Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells - PubMed (original) (raw)
. 2008 Sep 19;29(3):497-510.
doi: 10.1016/j.immuni.2008.07.013.
Rimpei Morita, Maochang Liu, Yanying Cao, Sebastien Coquery, Luann Thompson-Snipes, Francine Briere, Damien Chaussabel, Gerard Zurawski, A Karolina Palucka, Yoram Reiter, Jacques Banchereau, Hideki Ueno
Affiliations
- PMID: 18789730
- PMCID: PMC2688399
- DOI: 10.1016/j.immuni.2008.07.013
Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells
Eynav Klechevsky et al. Immunity. 2008.
Abstract
Little is known about the functional differences between the human skin myeloid dendritic cell (DC) subsets, epidermal CD207(+) Langerhans cells (LCs) and dermal CD14(+) DCs. We showed that CD14(+) DCs primed CD4(+) T cells into cells that induce naive B cells to switch isotype and become plasma cells. In contrast, LCs preferentially induced the differentiation of CD4(+) T cells secreting T helper 2 (Th2) cell cytokines and were efficient at priming and crosspriming naive CD8(+) T cells. A third DC population, CD14(-)CD207(-)CD1a(+) DC, which resides in the dermis, could activate CD8(+) T cells better than CD14(+) DCs but less efficiently than LCs. Thus, the human skin displays three DC subsets, two of which, i.e., CD14(+) DCs and LCs, display functional specializations, the preferential activation of humoral and cellular immunity, respectively.
Figures
Figure 1
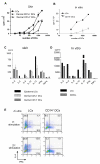
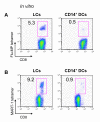
Purification and characterization of dermal and epidermal DCs obtained from human skin and of in vitro CD34-HPCs derived mDC subsets (A) Expression of CD1a and CD14 on purified skin DCs defines epidermal CD1a+ LCs, dermal CD1a+ DCs, and dermal CD14+ DCs subpopulations. (B) Expression of CD1a and CD14 on CD34-HPC cultured with GM-CSF and TNFα for 9 days defines two subpopulations of mDCs, LCs and CD14+ DCs. (C) Expression of surface antigens on skin and in vitro-generated DC subsets. Data show similarity of in vitro LCs to skin LCs, and of the in vitro CD14+ DCs to skin dermal CD14+ DCs. Dermal CD1a+ DCs show an intermediate phenotype compared to LCs and dermal CD14+ DCs. (D) Cytokine production by CD40L-activated skin DC subsets. Data indicate the expression of IL-15 by LCs and dermal CD1a+ DCs and IL-10 production by dermal CD14+ DCs. Production of IL-6, IL12p40, MCP-1, GM-CSF, IL-1β and TNF-α is restricted to CD14+ DCs.
Figure 2
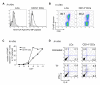
LCs are superior to CD14+ DCs at inducing the proliferation of allogeneic naïve CD4+ T cells and the differentiation of Type 2 cytokines secreting CD4+ T cells (A) Skin-LCs induce more robust proliferation of allogeneic naïve CD4+ T cells than do dermal CD1a+ DCs or dermal CD14+ DCs. [H3]-thymidine incorporation. One of four experiments. (B) In vitro-generated LCs stimulate stronger proliferation of allogeneic naïve CD4+ T cells than do CD14+ DCs. One five experiments. (C) Naive CD4+ T cells primed with skin LCs secrete more Type 2 cytokines than those primed with dermal DC subsets. Proliferating naïve CD4+ T cells, primed by skin mDC subsets, were sorted and re-stimulated with CD3/CD28 mAbs overnight followed by cytokine analysis. One of three experiments. (D) Naive CD4+ T cells primed with in vitro-generated LCs secrete more Type 2 cytokines upon restimulation. One of five experiments. (E) LCs promote T cell differentiation towards IFNγ-IL-4+ Type 2 T cells. Naïve CD4+ T cells cultured for 6 d with each in vitro-generated mDC subsets were stimulated with PMA and ionomycin in the presence of monensin. Intracytoplasmic cytokines were analyzed using anti-IFNγ and anti-IL-4 mAbs (upper panel). Some CD4+ T cells were restimulated with the same DC subset for 3 days before the cytokine analysis (lower panel). One of ten experiments.
Figure 3
CD14+ DCs but not LCs or dermal CD1a+ DCs polarize CD4+ T cells into Tfh cells (A) Experimental protocol. Allogeneic naïve CD4+ T cells were cultured for 7 days with sorted purified mDC subsets. Activated CD4+ T cells (FSChighCD11c-CD4+ T cells) were sorted and cultured with naïve B cells that were preactivated with CpG and anti-IgM. After 14 days, Ig levels were measured by ELISA. (B) Ig production by naïve B cells cocultured with CD4+ T cells primed with distinct skin mDC subsets. Naïve B cells were cultured with indicated number of CD4+ T cells stimulated by each skin mDC subset. (C) Ig production assay with in vitro-generated mDC subsets. (D-E) Production of CXCL13 by CD4+ T cells primed by skin mDC subsets (D) or in vitro-generated mDC subsets (E). Primed CD4+ T cells (day 7) were restimulated overnight with CD3/CD28 mAbs before CXCL13 was measured.
Figure 4
LCs are more efficient than CD14+ DCs at priming allogeneic naïve CD8+ T cells (A) Skin LCs stimulate stronger proliferation of allogeneic naïve CD8+ T cells than do dermal CD1a+ DCs or dermal CD14+ DCs. [H3]-thymidine incorporation. One of four experiments. (B) In vitro-generated LCs stimulate stronger proliferation of allogeneic naïve CD8+ T cells than do CD14+ DCs. One of four experiments. (C) Skin LCs stimulate stronger proliferation of allogeneic naïve CD8+ T cells than do dermal CD1a+ DCs or dermal CD14+ DCs as indicated by dilution of CFSE dye. One of three experiments.
Figure 5
LCs are more efficient than CD14+ DCs at priming antigen specific high avidity effector CD8+ T cells (A) LCs from an HLA-A201+ donor, loaded with an HLA-A201-restricted MART-1 peptide, induce higher expansion of specific CD8+ T cells when compared to peptide loaded-CD14+ DCs (staining with MART-1-HLA-A201 tetramer). (B) MART-1-specific CD8+ T cells primed by LCs and CD14+ DCs are cytotoxic against T2 cells loaded with 10-8 M MART-126-35 peptide at high Effector to Target (E:T) ratio (30:1, left panel). At a lower E:T ratio (4:1. right panel), LCs-primed CTLs are more cytotoxic than CD14+ DCs-primed CTLs. (C) MART-1-specific CD8+ T cells primed by LCs, but not by CD14+ DCs, can kill the HLA A201+ melanoma cell line MEL526 expressing MART-1. (D) MART-1-HLA-A201 tetramer fluorescence intensity is higher on CD8+ T cells primed by LCs than on those primed by CD14+ DCs. fifteen experiments. Paired Student t-test. (E) MART-1-HLA-A201 tetramers dissociate at a slower rate from CD8+ T cells primed by LCs than from those primed by CD14+ DCs. CD8+ T cells primed by MART-126-35-loaded mDC subsets were incubated with MART-1/HLA-A201 tetramer, and then an excess of unlabeled anti-HLA-A201 Ab was added to prevent rebinding of the tetramer after dissociation. The intensity of tetramer fluorescence was analyzed at various time points. Data are shown as the natural logarithm of the percentage of maximum fluorescence (corresponding to mean fluorescence at _t_0) plotted against time. (F) Staining peptide-loaded DCs with monoclonal antibodies endowed with TCR-like specificity, revealed that CD14+ DCs present higher levels of peptide-HLA-A201 complexes than LCs upon loading with HLA-A201 peptides (MART-126-35 upper panel, gp100209-217 in lower panel). (G) LCs-primed CD8+ T cells express higher level of the effector molecules; granzyme A, Granzyme B and perforin compared to dermal DC subsets CD1a+-and CD14+ DCs.
Figure 6
LCs are more efficient than CD14+ DCs at cross-presenting antigens to CD8+ T cells (A) LCs loaded with soluble Flu-MP efficiently cross-present and expand Flu-MP58-66-specific CD8+ T cells when compared to CD14+ DCs. (B) LCs cross-prime MART-1-specific CD8+ T cells more efficiently than CD14+ DCs when loaded with a 15-mer MART-1 peptide (MART-121-35) as analysed by MART-126-35-HLA-A201 tetramer staining.
Figure 7
Both CD14+ DCs and LCs efficiently activate memory T cells (A) Flu-MP58-66 peptide-loaded CD14+ DCs express more Flu-MP58-66/HLA-A201 complexes on the cell surface than peptide-loaded LCs, as determined with a Flu-MP58-66/HLA-A201-specific tetramerized monoclonal antibody. (B) Frequency of Flu-MP58-66-specific CD8+ T cells analyzed with Flu-MP58-66-HLA-A201 tetramer 9 days after activation of CD8+ T cells with Flu-MP peptide-loaded LCs or CD14+ DCs from an HLA-A201+ donor. (C) Kinetics of Flu-MP58-66-specific CD8+ T cell expansion in response to stimulation with peptide-loaded LCs or CD14+ DCs. (D) CD14+ DCs efficiently stimulate autologous TT-specific memory CD4+ T cells. CD4+ T cells were stimulated with each autologous DC subset loaded with TT or none. At day 7, T cells were restimulated with CD34-DCs loaded with TT for 5 h, and intracytoplasmic IFNγ and IL-4 production was analyzed by flow cytometry. One of four experiments.
References
- Allan RS, Smith CM, Belz GT, van Lint AL, Wakim LM, Heath WR, Carbone FR. Epidermal viral immunity induced by CD8alpha+ dendritic cells but not by Langerhans cells. Science. 2003;301:1925–1928. - PubMed
- Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. - PubMed
- Alvarez D, Harder G, Fattouh R, Sun J, Goncharova S, Stampfli MR, Coyle AJ, Bramson JL, Jordana M. Cutaneous antigen priming via gene gun leads to skin-selective Th2 immune-inflammatory responses. J Immunol. 2005;174:1664–1674. - PubMed
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y, Pulendran B, Palucka K. Immunobiology of dendritic cells. Ann Rev Immunol. 2000;18:767–811. - PubMed
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P0-1 CA84512/CA/NCI NIH HHS/United States
- R0-1 CA85540/CA/NCI NIH HHS/United States
- R01 CA085540/CA/NCI NIH HHS/United States
- P01 CA084512/CA/NCI NIH HHS/United States
- U19 AI057234/AI/NIAID NIH HHS/United States
- U-19 AI-57234/AI/NIAID NIH HHS/United States
- R01 CA078846/CA/NCI NIH HHS/United States
- R0-1 CA78846/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials