An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer - PubMed (original) (raw)
doi: 10.1158/1535-7163.MCT-08-0573.
Volker Fendrich, Karen McGovern, Djahida Bedja, Savita Bisht, Hector Alvarez, Jan-Bart M Koorstra, Nils Habbe, Collins Karikari, Michael Mullendore, Kathleen L Gabrielson, Rajni Sharma, William Matsui, Anirban Maitra
Affiliations
- PMID: 18790753
- PMCID: PMC2605523
- DOI: 10.1158/1535-7163.MCT-08-0573
An orally bioavailable small-molecule inhibitor of Hedgehog signaling inhibits tumor initiation and metastasis in pancreatic cancer
Georg Feldmann et al. Mol Cancer Ther. 2008 Sep.
Abstract
Recent evidence suggests that blockade of aberrant Hedgehog signaling can be exploited as a therapeutic strategy for pancreatic cancer. Our previous studies using the prototype Hedgehog small-molecule antagonist cyclopamine had shown the striking inhibition of systemic metastases on Hedgehog blockade in spontaneously metastatic orthotopic xenograft models. Cyclopamine is a natural compound with suboptimal pharmacokinetics, which impedes clinical translation. In the present study, a novel, orally bioavailable small-molecule Hedgehog inhibitor, IPI-269609, was tested using in vitro and in vivo model systems. In vitro treatment of pancreatic cancer cell lines with IPI-269609 resembled effects observed using cyclopamine (i.e., Gli-responsive reporter knockdown, down-regulation of the Hedgehog target genes Gli1 and Ptch, as well as abrogation of cell migration and colony formation in soft agar). Single-agent IPI-269609 profoundly inhibited systemic metastases in orthotopic xenografts established from human pancreatic cancer cell lines, although Hedgehog blockade had minimal effect on primary tumor volume. The only discernible phenotype observed within the treated primary tumor was a significant reduction in the population of aldehyde dehydrogenase-bright cells, which we have previously identified as a clonogenic tumor-initiating population in pancreatic cancer. Selective ex vivo depletion of aldehyde dehydrogenase-bright cells with IPI-269609 was accompanied by significant reduction in tumor engraftment rates in athymic mice. Pharmacologic blockade of aberrant Hedgehog signaling might prove to be an effective therapeutic strategy for inhibition of systemic metastases in pancreatic cancer, likely through targeting subsets of cancer cells with tumor-initiating ("cancer stem cell") properties.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
K. McGovern, Infinity Pharmaceuticals employee. Infinity Pharmaceuticals donated cyclopamine and IPI-269609 for this study and approved the manuscript prior to publication, as regulated by a material transfer agreement. IPI-269609 is covered by U.S. patent no. US 7,230,004 B2, owned by Infinity Discovery, Inc., Cambridge, MA. The other authors reported no potential conflicts of interest.
Figures
Figure 1
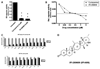
IPI-269609 inhibits Hedgehog signaling in vitro. In Gli-responsive reporter assays, IPI-269609 blocked the pathway activity of LightII cells stimulated with conditioned medium containing ShhN (A). (**, P < 0.01, compared with controls.) In LightII reporter assays, IPI-269609 showed a dose-response curve similar to that of cyclopamine (B). Hedgehog pathway inhibition led to a wide range of in vitro growth inhibition as observed by 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assays in a panel of human pancreatic cancer cell lines (C). IPI-269609 is a semisynthetic analogue of the plant-derived natural product cyclopamine. IPI-269609 has a molecular weight of 423 Da and differs from cyclopamine in that it has a seven-member D-ring and a conjugated A-ring ketone. The combination of these structural modifications is responsible for its enhanced pharmaceutical properties relative to cyclopamine (D).
Figure 2
Treatment of pancreatic cancer cell lines with IPI-269609 in vitro resembles the biological effects of Hedgehog pathway blockade. Similarly to cyclopamine, incubation with IPI-269609 (6 µmol/L) caused a marked down-regulation of Gli1 steady-state mRNA levels in the pancreatic cancer cell line E3LZ10.7 (A). In vitro treatment with IPI-269609 reduced cell migration as observed in wound assays in E3LZ10.7 and Capan-1 (B), as well as colony formation and anchorage-independent growth in soft agar (C). IPI-269609 reduced the fraction of SSC-low/ALDH-bright cells in the E3LZ10.7 cell line. Treatment with the ALDH inhibitor diethylamino-benzaldehyde (DEAB) served as a negative control (D). The graph shows a representative of three independent experiments (*, P < 0.05; **, P < 0.01).
Figure 3
Treatment of s.c. E3LZ10.7 xenografts with IPI-269609. Although after 25 d of drug treatment the average xenograft tumor size was ~40% smaller in IPI-269609 (20 mg/kg/d p.o.) treated animals as compared with controls, this difference was not statistically significant. Growth inhibition by gemcitabine (100 mg/kg i.p. every 4th day) was much more pronounced. There was no difference in growth inhibition between animals treated with gemcitabine alone and those treated with the combination of IPI-269609 plus gemcitabine (n = 4 per group). *, P < 0.05.
Figure 4
Treatment of orthotopic E3LZ10.7 xenografts with IPI-269609. A, treatment with IPI-269609 (20 mg/kg/d p.o.) for 30 d did not affect the growth of orthotopic E3LZ10.7 xenografts. B, H&E stainings of orthotopic E3LZ10.7 treated with IPI-269609 (i) or solvent only (ii ). C, examples of histologies of E3LZ10.7 metastases to lymph node (i) and spleen (ii ); H&E staining.
Figure 5
Application of IPI-269609 caused significant down-regulation of the Hedgehog target gene Ptch1 at the mRNA level in vivo in E3LZ10.7 xenografts (A), which was accompanied by reduction of cancer cells with high ALDH activity as visualized by immunohistochemistry (B). ALDH-positive cells were quantified using Frida analysis software (C; *, P < 0.05).
Figure 6
Peri-implantational Hedgehog blockade is sufficient to cause in vivo growth inhibition of pancreatic cancer xenografts. A, treatment scheme used: Hedgehog signaling in pancreatic cancer cell lines was suppressed by treatment with IPI-269609 in vitro for 3 d. At the same time, continuous application of IPI-269609 to nude mice using s.c. osmotic pumps was initiated and continued for another 4 d after implantation of tumor cells. Mock treatment with solvent only was used as control, and only cell suspensions with >95% viable cells were used for injection. S.c. tumor growth was measured 1 and 2 wk after cancer cell injection. Pretreatment with IPI-269609 led to significantly reduced growth of s.c. E3LZ10.7 and xenografts in nude mice (B; n = 6 per group). **, P < 0.01.
Similar articles
- Combination of hedgehog signaling blockage and chemotherapy leads to tumor reduction in pancreatic adenocarcinomas.
Bahra M, Kamphues C, Boas-Knoop S, Lippert S, Esendik U, Schüller U, Hartmann W, Waha A, Neuhaus P, Heppner F, Pietsch T, Koch A. Bahra M, et al. Pancreas. 2012 Mar;41(2):222-9. doi: 10.1097/MPA.0b013e31822896dd. Pancreas. 2012. PMID: 22076568 - Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers.
Feldmann G, Dhara S, Fendrich V, Bedja D, Beaty R, Mullendore M, Karikari C, Alvarez H, Iacobuzio-Donahue C, Jimeno A, Gabrielson KL, Matsui W, Maitra A. Feldmann G, et al. Cancer Res. 2007 Mar 1;67(5):2187-96. doi: 10.1158/0008-5472.CAN-06-3281. Cancer Res. 2007. PMID: 17332349 Free PMC article. - Polymeric nanoparticle-encapsulated hedgehog pathway inhibitor HPI-1 (NanoHHI) inhibits systemic metastases in an orthotopic model of human hepatocellular carcinoma.
Xu Y, Chenna V, Hu C, Sun HX, Khan M, Bai H, Yang XR, Zhu QF, Sun YF, Maitra A, Fan J, Anders RA. Xu Y, et al. Clin Cancer Res. 2012 Mar 1;18(5):1291-302. doi: 10.1158/1078-0432.CCR-11-0950. Epub 2011 Aug 25. Clin Cancer Res. 2012. PMID: 21868763 Free PMC article. - Agents targeting the Hedgehog pathway for pancreatic cancer treatment.
Bisht S, Brossart P, Maitra A, Feldmann G. Bisht S, et al. Curr Opin Investig Drugs. 2010 Dec;11(12):1387-98. Curr Opin Investig Drugs. 2010. PMID: 21154121 Review. - Blockade of hedgehog signaling pathway as a therapeutic strategy for pancreatic cancer.
Xu FG, Ma QY, Wang Z. Xu FG, et al. Cancer Lett. 2009 Oct 8;283(2):119-24. doi: 10.1016/j.canlet.2009.01.014. Epub 2009 Feb 15. Cancer Lett. 2009. PMID: 19232458 Review.
Cited by
- The role of radiotherapy in locally advanced pancreatic carcinoma.
Gutt R, Liauw SL, Weichselbaum RR. Gutt R, et al. Nat Rev Gastroenterol Hepatol. 2010 Aug;7(8):437-47. doi: 10.1038/nrgastro.2010.98. Epub 2010 Jul 13. Nat Rev Gastroenterol Hepatol. 2010. PMID: 20628346 Review. - The role of breast cancer stem cells in metastasis and therapeutic implications.
Velasco-Velázquez MA, Popov VM, Lisanti MP, Pestell RG. Velasco-Velázquez MA, et al. Am J Pathol. 2011 Jul;179(1):2-11. doi: 10.1016/j.ajpath.2011.03.005. Epub 2011 Apr 28. Am J Pathol. 2011. PMID: 21640330 Free PMC article. Review. - Casticin inhibits the epithelial-mesenchymal transition in ovarian carcinoma via the hedgehog signaling pathway.
Zhang J, Cui Y, Sun S, Cao J, Fang X. Zhang J, et al. Oncol Lett. 2018 Apr;15(4):4495-4502. doi: 10.3892/ol.2018.7880. Epub 2018 Jan 26. Oncol Lett. 2018. PMID: 29541219 Free PMC article. - Will a mAb-Based Immunotherapy Directed against Cancer Stem Cells Be Feasible?
Santamaria S, Delgado M, Kremer L, Garcia-Sanz JA. Santamaria S, et al. Front Immunol. 2017 Nov 9;8:1509. doi: 10.3389/fimmu.2017.01509. eCollection 2017. Front Immunol. 2017. PMID: 29170667 Free PMC article. Review. - Targeting Triple NK Cell Suppression Mechanisms: A Comprehensive Review of Biomarkers in Pancreatic Cancer Therapy.
Fanijavadi S, Thomassen M, Jensen LH. Fanijavadi S, et al. Int J Mol Sci. 2025 Jan 9;26(2):515. doi: 10.3390/ijms26020515. Int J Mol Sci. 2025. PMID: 39859231 Free PMC article. Review.
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
- Lohr JM. Medical treatment of pancreatic cancer. Expert Rev Anticancer Ther. 2007;7:533–544. - PubMed
- Ujiki MB, Talamonti MS. Guidelines for the surgical management of pancreatic adenocarcinoma. Semin Oncol. 2007;34:311–320. - PubMed
- Berman DM, Karhadkar SS, Maitra A, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical