Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress - PubMed (original) (raw)
Dicarbonyls linked to damage in the powerhouse: glycation of mitochondrial proteins and oxidative stress
Naila Rabbani et al. Biochem Soc Trans. 2008 Oct.
Abstract
Protection of mitochondrial proteins from glycation by endogenous dicarbonyl compounds, methylglyoxal and glyoxal, was found recently to prevent increased formation of reactive oxygen species and oxidative and nitrosative damage to the proteome during aging and produce life extension in the nematode Caenorhabditis elegans. This suggests that dicarbonyl glycation damage to the mitochondrial proteome may be a preceding event to mitochondrial dysfunction leading to oxidative stress. Future research will address the functional charges in mitochondrial proteins that are the targets for dicarbonyl glycation.
Figures
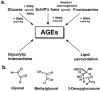
Figure 1. Major pathways for the formation of AGEs in physiological systems and precursor dicarbonyl metabolites
(a) Formation of early and advanced glycation adducts from glucose and glycolytic intermediates and products of lipid peroxidation. (b) Physiological reactive dicarbonyl-glycating agents.
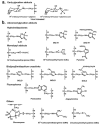
Figure 2. Protein glycation adduct residues
(a) Early glycation adducts. (b) AGEs. For the corresponding free adducts at physiological pH, the N-terminal amino group is protonated −NH3+ and the C-terminal carbonyl is a carboxylate −CO2− moiety. DOLD, 3-deoxyglucosone-derived lysine dimer; GOLD, glyoxal-derived lysine dimer; MOLD, methylglyoxal-derived lysine dimer.
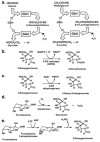
Figure 3. The enzymatic defence against glycation
(a) Metabolism of glyoxal and MG by the glyoxalase system. (b) Metabolism of 3DG by 3-deoxyglucosone reductase, an AKRd. (c) Metabolism of 3DG by 3-deoxyglucosone dehydrogenase. (d, e) Repair of early glycated proteins by Amadoriase and fructosamine-3-phosphokinase.
Similar articles
- [Glycation of mitochondrial proteins, oxidative stress and aging].
Naudí A, Jové M, Ayala V, Portero-Otín M, Pamplona R. Naudí A, et al. Rev Esp Geriatr Gerontol. 2010 May-Jun;45(3):156-66. doi: 10.1016/j.regg.2010.02.001. Epub 2010 Mar 26. Rev Esp Geriatr Gerontol. 2010. PMID: 20347183 Spanish. - The dicarbonyl proteome: proteins susceptible to dicarbonyl glycation at functional sites in health, aging, and disease.
Rabbani N, Thornalley PJ. Rabbani N, et al. Ann N Y Acad Sci. 2008 Apr;1126:124-7. doi: 10.1196/annals.1433.043. Ann N Y Acad Sci. 2008. PMID: 18448805 - Antioxidant and pro-oxidant actions of resveratrol on human serum albumin in the presence of toxic diabetes metabolites: Glyoxal and methyl-glyoxal.
Arcanjo NMO, Luna C, Madruga MS, Estévez M. Arcanjo NMO, et al. Biochim Biophys Acta Gen Subj. 2018 Sep;1862(9):1938-1947. doi: 10.1016/j.bbagen.2018.06.007. Epub 2018 Jun 11. Biochim Biophys Acta Gen Subj. 2018. PMID: 29902553 - Protein Glycation in Plants-An Under-Researched Field with Much Still to Discover.
Rabbani N, Al-Motawa M, Thornalley PJ. Rabbani N, et al. Int J Mol Sci. 2020 May 30;21(11):3942. doi: 10.3390/ijms21113942. Int J Mol Sci. 2020. PMID: 32486308 Free PMC article. Review. - Mitochondrial respiration and reactive oxygen species in C. elegans.
Sedensky MM, Morgan PG. Sedensky MM, et al. Exp Gerontol. 2006 Oct;41(10):957-67. doi: 10.1016/j.exger.2006.06.056. Epub 2006 Aug 21. Exp Gerontol. 2006. PMID: 16919906 Review.
Cited by
- Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging.
Fournet M, Bonté F, Desmoulière A. Fournet M, et al. Aging Dis. 2018 Oct 1;9(5):880-900. doi: 10.14336/AD.2017.1121. eCollection 2018 Oct. Aging Dis. 2018. PMID: 30271665 Free PMC article. Review. - GATD3A, a mitochondrial deglycase with evolutionary origins from gammaproteobacteria, restricts the formation of advanced glycation end products.
Smith AJ, Advani J, Brock DC, Nellissery J, Gumerson J, Dong L, Aravind L, Kennedy B, Swaroop A. Smith AJ, et al. BMC Biol. 2022 Mar 21;20(1):68. doi: 10.1186/s12915-022-01267-6. BMC Biol. 2022. PMID: 35307029 Free PMC article. - Overexpression of glyoxalase-I reduces hyperglycemia-induced levels of advanced glycation end products and oxidative stress in diabetic rats.
Brouwers O, Niessen PM, Ferreira I, Miyata T, Scheffer PG, Teerlink T, Schrauwen P, Brownlee M, Stehouwer CD, Schalkwijk CG. Brouwers O, et al. J Biol Chem. 2011 Jan 14;286(2):1374-80. doi: 10.1074/jbc.M110.144097. Epub 2010 Nov 5. J Biol Chem. 2011. PMID: 21056979 Free PMC article. - Modulation of the glyoxalase system in the aging model Podospora anserina: effects on growth and lifespan.
Scheckhuber CQ, Mack SJ, Strobel I, Ricciardi F, Gispert S, Osiewacz HD. Scheckhuber CQ, et al. Aging (Albany NY). 2010 Dec;2(12):969-80. doi: 10.18632/aging.100251. Aging (Albany NY). 2010. PMID: 21212464 Free PMC article. - Weak association of glyoxalase 1 (GLO1) variants with autism spectrum disorder.
Kovač J, Podkrajšek KT, Lukšič MM, Battelino T. Kovač J, et al. Eur Child Adolesc Psychiatry. 2015 Jan;24(1):75-82. doi: 10.1007/s00787-014-0537-8. Epub 2014 Mar 27. Eur Child Adolesc Psychiatry. 2015. PMID: 24671236
References
- Morcos M, Du X, Pfisterer F, Hutter H, Sayed AAR, Thornalley P, Ahmed N, Baynes J, Thorpe S, Kukudov G, et al. Glyoxalase-1 prevents mitochondrial protein modification and enhances lifespan in Caenorhabditis elegans. Aging Cell. 2008;7:260–269. - PubMed
- Calabrese V, Guagliano E, Sapienza M, Panebianco M, Calafato S, Puleo E, Pennisi G, Mancuso C, Butterfield DA, Stella AG. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem. Res. 2007;32:757–773. - PubMed
- Thornalley PJ. The glyoxalase system in health and disease. Mol. Aspects Med. 1993;14:287–371. - PubMed
- Thornalley PJ. The clinical significance of glycation. Clin. Lab. 1999;45:263–273.
Publication types
MeSH terms
Substances
Grants and funding
- A5147/CRUK_/Cancer Research UK/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- BHF_/British Heart Foundation/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources