Translational Research Working Group developmental pathway for immune response modifiers - PubMed (original) (raw)
Translational Research Working Group developmental pathway for immune response modifiers
Martin A Cheever et al. Clin Cancer Res. 2008.
Abstract
The Translational Research Working Group (TRWG) was created as a national initiative to evaluate the current status of the investment of National Cancer Institute in translational research and envision its future. The Translational Research Working Group conceptualized translational research as a set of six developmental processes or pathways focused on various clinical goals. One of those pathways describes the development of immune response modifiers such as vaccines and cytokines. A hallmark of the Immune Response Modifier Developmental Pathway is the coordinated development of multiple components. The Immune Response Modifier Pathway was conceived not as a comprehensive description of the corresponding real-world processes but rather as a tool designed to facilitate movement of a candidate assay through the translational process to the point where it can be handed off for definitive clinical testing. This paper discusses key challenges associated with the immune response modifier agent development process in light of the pathway.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures
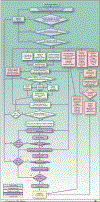
Fig. 1.
Immune Response Modifiers (IRM) Pathway. The IRM pathway is depicted as a flowchart, a schematic process representation widely used in engineering. Rounded rectangle at the top, origin of the process. Square-cornered rectangles, activity steps. Diamonds, conditional tests or decision steps. Unidirectional arrows, the direction of the activity sequence, and the direction of transfer of supporting tools from their parallel development paths to the main path of modality development. Bidirectional arrows, codevelopment or concurrent, interactive refinement. The initial steps of the pathway (blue) are required to proceed through the pathway, with the blue diamonds representing the credentialing steps of scientific validation, clinical need, and feasibility. The pathway proceeds to multiple parallel paths representing development of multiple components of the modality itself (green) as well as development of different classes of supporting tools (red). The three green boxes at the top of the creation of the modality path acknowledges the parallel and interactive development of a multicompent formulation and/or regimen, such as the need for an antigen, a delivery vehicle, and an immune modulator for the development of a cancer vaccine. The red boxes at the top of the supporting tools path depict the development of assays for characterizing and evaluating the effects of the modality and for defining the cohort for which the modality is appropriate. The development of these tools is further detailed in the Biospecimen-based or Imaging-based Assessment Modality pathway. Parallel paths have been made explicit to acknowledge that some of the required tools may not exist, and their parallel or codevelopment will be prerequisite for the viability of the new modality. Subsequent steps include preclinical development (purple) and early stage clinical trials (yellow). For each activity, decision point, parallel path, or feedback loop, it is understood that there are many more variations that can occur, and that not all steps may occur in each instance. The pathway does not address the ways in which insights gained from late-stage clinical trials can influence the development process. Immune response modifier agent interventions may be used for treatment or for primary, secondary, or tertiary prevention. The pathways are conceived not as comprehensive descriptions of the corresponding real-world processes but as tools designed to serve specific purposes, including research program and project management, coordination of research efforts, and professional and lay education and communication.
Similar articles
- The Translational Research Working Group developmental pathway for anticancer agents (drugs or biologics).
Schilsky RL, Gordon G, Gilmer TM, Courtneidge SA, Matrisian LM, Grad O, Nelson WG; Translational Research Working Group. Schilsky RL, et al. Clin Cancer Res. 2008 Sep 15;14(18):5685-91. doi: 10.1158/1078-0432.CCR-08-1265. Clin Cancer Res. 2008. PMID: 18794076 Free PMC article. - Translational Research Working Group developmental pathway for biospecimen-based assessment modalities.
Srivastava S, Gray JW, Reid BJ, Grad O, Greenwood A, Hawk ET; Translational Research Working Group. Srivastava S, et al. Clin Cancer Res. 2008 Sep 15;14(18):5672-7. doi: 10.1158/1078-0432.CCR-08-1267. Clin Cancer Res. 2008. PMID: 18794074 Free PMC article. - The Translational Research Working Group developmental pathway for lifestyle alterations.
Hawk ET, Greenwood A, Gritz ER, McTiernan A, Sellers T, Hursting SD, Leischow S, Grad O; Translational Research Working Group. Hawk ET, et al. Clin Cancer Res. 2008 Sep 15;14(18):5707-13. doi: 10.1158/1078-0432.CCR-08-1262. Clin Cancer Res. 2008. PMID: 18794079 - [Current status and future perspective of cancer immunotherapy--clinical application and development].
Aruga A. Aruga A. Nihon Geka Gakkai Zasshi. 2013 Nov;114(6):327-31. Nihon Geka Gakkai Zasshi. 2013. PMID: 24358730 Review. Japanese. No abstract available. - [Immunological tumor therapy].
Dietrich K, Theobald M. Dietrich K, et al. Internist (Berl). 2015 Aug;56(8):907-16; quiz 917. doi: 10.1007/s00108-015-3744-6. Internist (Berl). 2015. PMID: 26187335 Review. German.
Cited by
- The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research.
Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT, Mellman I, Prindiville SA, Viner JL, Weiner LM, Matrisian LM. Cheever MA, et al. Clin Cancer Res. 2009 Sep 1;15(17):5323-37. doi: 10.1158/1078-0432.CCR-09-0737. Clin Cancer Res. 2009. PMID: 19723653 Free PMC article. - Immunotherapy in prostate cancer: emerging strategies against a formidable foe.
Bilusic M, Heery C, Madan RA. Bilusic M, et al. Vaccine. 2011 Sep 2;29(38):6485-97. doi: 10.1016/j.vaccine.2011.06.088. Epub 2011 Jul 7. Vaccine. 2011. PMID: 21741424 Free PMC article. Review. - Influence of tumour micro-environment heterogeneity on therapeutic response.
Junttila MR, de Sauvage FJ. Junttila MR, et al. Nature. 2013 Sep 19;501(7467):346-54. doi: 10.1038/nature12626. Nature. 2013. PMID: 24048067 Review. - Role of T lymphocytes in tumor response to radiotherapy.
Demaria S, Formenti SC. Demaria S, et al. Front Oncol. 2012 Aug 24;2:95. doi: 10.3389/fonc.2012.00095. eCollection 2012. Front Oncol. 2012. PMID: 22937524 Free PMC article. - Translational spinal cord injury research: preclinical guidelines and challenges.
Reier PJ, Lane MA, Hall ED, Teng YD, Howland DR. Reier PJ, et al. Handb Clin Neurol. 2012;109:411-33. doi: 10.1016/B978-0-444-52137-8.00026-7. Handb Clin Neurol. 2012. PMID: 23098728 Free PMC article. Review.
References
- Hawk ET, Matrisian LM, Nelson WG, et al. Translational Research Working Group developmental pathways: introduction and overview. Clin Cancer Res 2008;14: 5664–71. - PubMed
- Mihich E Combined effects of chemotherapy and immunity against Leukemia L1210 in DBA/2 Mice. Cancer Res 1969;29:848–54. - PubMed
- Arlen PM, Gulley JL, Madan RA, Hodge JW, Schlom J. Preclinical and clinical studies of recombinant poxvirus vaccines for carcinoma therapy. Crit Rev Immunol 2007;27:451–62. - PubMed
- Finn OJ. Cancer Immunology. N Engl J Med 2008; 58:2704–15. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources