A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells - PubMed (original) (raw)
. 2008 Sep 15;14(18):5759-68.
doi: 10.1158/1078-0432.CCR-08-0377.
Mohamed K Abou-Ghazal, Jun Wei, Arup Chakraborty, Wei Sun, Wei Qiao, Gregory N Fuller, Izabela Fokt, Elizabeth A Grimm, Robert J Schmittling, Gary E Archer Jr, John H Sampson, Waldemar Priebe, Amy B Heimberger
Affiliations
- PMID: 18794085
- PMCID: PMC2583362
- DOI: 10.1158/1078-0432.CCR-08-0377
A novel inhibitor of signal transducers and activators of transcription 3 activation is efficacious against established central nervous system melanoma and inhibits regulatory T cells
Ling-Yuan Kong et al. Clin Cancer Res. 2008.
Abstract
Purpose: Activation of signal transducers and activators of transcription 3 (STAT3) has been identified as a central mediator of melanoma growth and metastasis. We hypothesized that WP1066, a novel STAT3 blockade agent, has marked antitumor activity, even against the melanoma metastasis to brain, a site typically refractory to therapies.
Experimental design: The antitumor activities and related mechanisms of WP1066 were investigated both in vitro on melanoma cell lines and in vivo on mice with subcutaneously syngeneic melanoma or with intracerebral melanoma tumors.
Results: WP1066 achieved an IC(50) of 1.6, 2.3, and 1.5 mumol/L against melanoma cell line A375, B16, and B16EGFRvIII, respectively. WP1066 suppressed the phosphorylation of Janus-activated kinase 2 and STAT3 (Tyr705) in these cells. Tumor growth in mice with subcutaneously established syngeneic melanoma was markedly inhibited by WP1066 compared with that in controls. Long-term survival (>78 days) was observed in 80% of mice with established intracerebral syngeneic melanoma treated with 40 mg/kg of WP1066 in contrast to control mice who survived for a median of 15 days. Although WP1066 did not induce immunologic memory or enhance humoral responses to EGFRvIII, this compound reduced the production of immunosuppressive cytokines and chemokines (transforming growth factor-beta, RANTES, MCP-1, vascular endothelial growth factor), markedly inhibited natural and inducible Treg proliferation, and significantly increased cytotoxic immune responses of T cells.
Conclusions: The antitumor cytotoxic effects of WP1066 and its ability to induce antitumor immune responses suggest that this compound has potential for the effective treatment of melanoma metastatic to brain.
Figures
Figure 1
Western blot analyses showing that WP1066 suppresses the intracellular signaling of p-JAK2, p-STAT3, c-Myc, and survivin in melanoma cells. A, A375, B16, or B16EGFRvIII cells were cultured with either medium alone or medium supplemented with 5 μM of WP1066 for 2 h. Cell lysates were prepared and quantified for protein content. A total of 40 μg protein from each sample was used for immunoprecipitation and immunoblotting analysis of phospho-JAK2. Antiphosphotyrosine 4G10 conjugated to agarose beads was used for immunoprecipitation, and anti-JAK2 antibody was used for Western blotting. B, A375 cells were incubated with WP1066 at concentrations of 0, 1.25, 2.5, and 5 μM for 2 h. C, B16 or B16EGFRvIII cells were cultured with either medium alone or medium supplemented with 5 μM of WP1066 for 2 h. D, B16EGFRvIII cells were cultured with 5 μM of WP1066 for 0, 2, 4, 8, 12, 24 h. A total of 20 μg protein was electrophoretically fractionated in 8% SDS-polyacrylamide gels, transferred to nitrocellulose membranes, and followed by immunoblot analysis with specific antibodies against phospho-STAT3 (Tyr705), total STAT3, c-Myc, survivin, and β-Actin. In addition, the transferred membranes from B16 and B16EGFRvIII cell lysates were immunoblotted with a specific antibody against phospho-Akt (Ser473) or EGFRvIII.
Figure 2
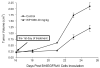
Volume of subcutaneous B16EGFRvIII tumors in C57BL/6J mice treated with vehicle control or with WP1066 via o.g. q.i.d. after tumors were measurable (n=6/group/experiment). On day 25, P < 0.05 comparing the WP1066-treated group to the vehicle control-treated group. The figure is the result of a single experiment, but it was performed twice.
Figure 3
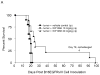
Survival data from C57BL/6J mice treated with WP1066 after intracerebral B16EGFRvIII cells or B16 cells were established in the brain. A, Antitumor efficacy was observed in mice injected i.p. with WP1066 (n=10 per dose), but it was dose limiting owing to localized inflammation. A comparison of survival time in the group treated i.p. with WP1066 at 20mg/kg with that for the vehicle control group yielded P values of 0.027, 0.011, 0.005, 0.002, and 0.001 on days 30, 35, 40, 45, and 50, respectively. B, C57BL/6J mice with established intracerebral B16EGFRvIII cells treated with WP1066 via oral gavage (n=10) showed at least a 324% increase in their median survival time, and 80% achieved long-term survival compared with those in the vehicle-treated controls (n=10). For the group treated with 40 mg/kg WP1066 by o.g., the P values were 0.04, 0.18, 0.007, 0.002, 0.001, and 0.001 at days 25, 30. 35, 40, 45, and 50, respectively, compared with the vehicle control group. C, Antitumor efficacy was also observed in C57BL/6J mice with established intracerebral B16 cells treated with WP1066 via oral gavage (n=10). In animals that survived longer than 78 days, subsequent rechallenge by injection of tumor cells into the contralateral hemisphere indicated that minimal immunological memory was induced (A, B, C).
Figure 3
Survival data from C57BL/6J mice treated with WP1066 after intracerebral B16EGFRvIII cells or B16 cells were established in the brain. A, Antitumor efficacy was observed in mice injected i.p. with WP1066 (n=10 per dose), but it was dose limiting owing to localized inflammation. A comparison of survival time in the group treated i.p. with WP1066 at 20mg/kg with that for the vehicle control group yielded P values of 0.027, 0.011, 0.005, 0.002, and 0.001 on days 30, 35, 40, 45, and 50, respectively. B, C57BL/6J mice with established intracerebral B16EGFRvIII cells treated with WP1066 via oral gavage (n=10) showed at least a 324% increase in their median survival time, and 80% achieved long-term survival compared with those in the vehicle-treated controls (n=10). For the group treated with 40 mg/kg WP1066 by o.g., the P values were 0.04, 0.18, 0.007, 0.002, 0.001, and 0.001 at days 25, 30. 35, 40, 45, and 50, respectively, compared with the vehicle control group. C, Antitumor efficacy was also observed in C57BL/6J mice with established intracerebral B16 cells treated with WP1066 via oral gavage (n=10). In animals that survived longer than 78 days, subsequent rechallenge by injection of tumor cells into the contralateral hemisphere indicated that minimal immunological memory was induced (A, B, C).
Figure 3
Survival data from C57BL/6J mice treated with WP1066 after intracerebral B16EGFRvIII cells or B16 cells were established in the brain. A, Antitumor efficacy was observed in mice injected i.p. with WP1066 (n=10 per dose), but it was dose limiting owing to localized inflammation. A comparison of survival time in the group treated i.p. with WP1066 at 20mg/kg with that for the vehicle control group yielded P values of 0.027, 0.011, 0.005, 0.002, and 0.001 on days 30, 35, 40, 45, and 50, respectively. B, C57BL/6J mice with established intracerebral B16EGFRvIII cells treated with WP1066 via oral gavage (n=10) showed at least a 324% increase in their median survival time, and 80% achieved long-term survival compared with those in the vehicle-treated controls (n=10). For the group treated with 40 mg/kg WP1066 by o.g., the P values were 0.04, 0.18, 0.007, 0.002, 0.001, and 0.001 at days 25, 30. 35, 40, 45, and 50, respectively, compared with the vehicle control group. C, Antitumor efficacy was also observed in C57BL/6J mice with established intracerebral B16 cells treated with WP1066 via oral gavage (n=10). In animals that survived longer than 78 days, subsequent rechallenge by injection of tumor cells into the contralateral hemisphere indicated that minimal immunological memory was induced (A, B, C).
Figure 4
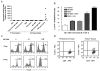
The production of TGF-β, MCP-1, RANTES, and VEGF is inhibited by WP1066 at doses below the IC50. B16 and B16EGFRvIII cells in logarithmic growth were treated with titered doses of WP1066 for 24 h. At a dose of 0.01 μM WP1066, the cytokines TGF-β, RANTES, VEGF, and the Treg chemokine MCP-1 were all inhibited, as detected in ELISA assays.
Figure 5
A, Humoral responses were not induced in mice vaccinated with PEP-3-KLH and WP1066 but were induced, as anticipated, with PEP-3-KLH and CFA. B, Cytotoxicity of the B16EGFRvIII cells in vitro produced by splenocytes from mice vaccinated with PEP-3-KLH or with PEP-3-KLH plus WP1066. The splenocyte effector cells from mice that were vaccinated with PEP-3-KLH induced minimal lysis. However, splenocyte effector cells from mice that were vaccinated with PEP-3-KLH plus WP1066 potently enhanced EGFRvIII-specific lysis (P< 0.05). Error bars show one standard deviation from mean values. C, WP1066 inhibits FoxP3 induction in T cells in peripheral blood and down regulates FoxP3 in natural Tregs. CD4+CD25-CD62Lhi naïve T cells from C57BL/6J mice were stimulated by plate-bound anti-CD3 (2 ug/ml) and soluble anti-CD28 (2 ug/ml) in the presence of TGFβ1 (1 ng/ml) and hIL-2 (200 U/ml) with 0, 0.1, and 1.0 μM WP1066 for inducible Tregs (iTreg) differentiation; CD4+CD25+ T cells (natural Tregs, nTreg) were stimulated by plate-bound anti-CD3 (2 ug/ml) and soluble anti-CD28 (2 ug/ml) in the presence of hIL-2 (200U/ml) with 0, 0.1, and 1.0 μM WP1066. Ninety-six hours after stimulation, the cells were analyzed for intracellular FoxP3 expression by flow cytometry. The percentage numbers for the indicated population are shown. D, FACS staining of peritumoral and tumor tissue isolated from a patient with melanoma metastasis to the brain. CD4+ cells were gated from the total cell population and plotted against FoxP3.
Similar articles
- The tumor microenvironment expression of p-STAT3 influences the efficacy of cyclophosphamide with WP1066 in murine melanoma models.
Hatiboglu MA, Kong LY, Wei J, Wang Y, McEnery KA, Fuller GN, Qiao W, Davies MA, Priebe W, Heimberger AB. Hatiboglu MA, et al. Int J Cancer. 2012 Jul 1;131(1):8-17. doi: 10.1002/ijc.26307. Epub 2011 Aug 24. Int J Cancer. 2012. PMID: 21792892 Free PMC article. - A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells.
Kong LY, Wei J, Sharma AK, Barr J, Abou-Ghazal MK, Fokt I, Weinberg J, Rao G, Grimm E, Priebe W, Heimberger AB. Kong LY, et al. Cancer Immunol Immunother. 2009 Jul;58(7):1023-32. doi: 10.1007/s00262-008-0618-y. Epub 2008 Nov 11. Cancer Immunol Immunother. 2009. PMID: 19002459 Free PMC article. - STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma.
Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K, Hayakawa M, Sumitomo M, Asano T. Horiguchi A, et al. Br J Cancer. 2010 May 25;102(11):1592-9. doi: 10.1038/sj.bjc.6605691. Epub 2010 May 11. Br J Cancer. 2010. PMID: 20461084 Free PMC article. - Preclinical characterization of signal transducer and activator of transcription 3 small molecule inhibitors for primary and metastatic brain cancer therapy.
Assi HH, Paran C, VanderVeen N, Savakus J, Doherty R, Petruzzella E, Hoeschele JD, Appelman H, Raptis L, Mikkelsen T, Lowenstein PR, Castro MG. Assi HH, et al. J Pharmacol Exp Ther. 2014 Jun;349(3):458-69. doi: 10.1124/jpet.114.214619. Epub 2014 Apr 2. J Pharmacol Exp Ther. 2014. PMID: 24696041 Free PMC article. - The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor.
Heimberger AB, Kong LY, Abou-Ghazal M, Reina-Ortiz C, Yang DS, Wei J, Qiao W, Schmittling RJ, Archer GE, Sampson JH, Hiraoka N, Priebe W, Fuller GN, Sawaya R. Heimberger AB, et al. Clin Neurosurg. 2009;56:98-106. Clin Neurosurg. 2009. PMID: 20214040 Review. No abstract available.
Cited by
- Degrasyn potentiates the antitumor effects of bortezomib in mantle cell lymphoma cells in vitro and in vivo: therapeutic implications.
Pham LV, Tamayo AT, Li C, Bornmann W, Priebe W, Ford RJ. Pham LV, et al. Mol Cancer Ther. 2010 Jul;9(7):2026-36. doi: 10.1158/1535-7163.MCT-10-0238. Epub 2010 Jul 6. Mol Cancer Ther. 2010. PMID: 20606045 Free PMC article. - IL-7 receptor blockade inhibits IL-17-producing γδ cells and suppresses melanoma development.
Li J, Liu J, Mao X, Tang Q, Lu H. Li J, et al. Inflammation. 2014 Oct;37(5):1444-52. doi: 10.1007/s10753-014-9869-2. Inflammation. 2014. PMID: 24619454 - Therapeutic targeting of anoikis resistance in cutaneous melanoma metastasis.
Neuendorf HM, Simmons JL, Boyle GM. Neuendorf HM, et al. Front Cell Dev Biol. 2023 Apr 26;11:1183328. doi: 10.3389/fcell.2023.1183328. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37181747 Free PMC article. Review. - The therapeutic potential of inhibitors of the signal transducer and activator of transcription 3 for central nervous system malignancies.
Heimberger AB. Heimberger AB. Surg Neurol Int. 2011;2:163. doi: 10.4103/2152-7806.89886. Epub 2011 Nov 14. Surg Neurol Int. 2011. PMID: 22140648 Free PMC article. - Degrasyn-like symmetrical compounds: possible therapeutic agents for multiple myeloma (MM-I).
Peng Z, Maxwell DS, Sun D, Bhanu Prasad BA, Schuber PT Jr, Pal A, Ying Y, Han D, Gao L, Wang S, Levitzki A, Kapuria V, Talpaz M, Young M, Showalter HD, Donato NJ, Bornmann WG. Peng Z, et al. Bioorg Med Chem. 2014 Feb 15;22(4):1450-8. doi: 10.1016/j.bmc.2013.12.048. Epub 2014 Jan 3. Bioorg Med Chem. 2014. PMID: 24457091 Free PMC article.
References
- Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. - PubMed
- Huang S. Regulation of metastases by signal transducer and activator of transcription 3 signaling pathway: clinical implications. Clin Cancer Res. 2007;13:1362–6. - PubMed
- Xie TX, Wei D, Liu M, et al. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene. 2004;23:3550–60. - PubMed
- Lo HW, Hsu SC, Ali-Seyed M, et al. Nuclear interaction of EGFR and STAT3 in the activation of the iNOS/NO pathway. Cancer Cell. 2005;7:575–89. - PubMed
- Haura EB, Zheng Z, Song L, Cantor A, Bepler G. Activated epidermal growth factor receptor-Stat-3 signaling promotes tumor survival in vivo in non-small cell lung cancer. Clin Cancer Res. 2005;11:8288–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA120813/CA/NCI NIH HHS/United States
- CA120813-01/CA/NCI NIH HHS/United States
- A1077225-01/PHS HHS/United States
- R01 CA120813-01A1/CA/NCI NIH HHS/United States
- P50 CA093459-01A1/CA/NCI NIH HHS/United States
- P50 CA093459/CA/NCI NIH HHS/United States
- R43 AI077225-01/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous