PI3K pathway alterations in cancer: variations on a theme - PubMed (original) (raw)
Review
PI3K pathway alterations in cancer: variations on a theme
T L Yuan et al. Oncogene. 2008.
Abstract
The high frequency of phosphoinositide 3-kinase (PI3K) pathway alterations in cancer has led to a surge in the development of PI3K inhibitors. Many of these targeted therapies are currently in clinical trials and show great promise for the treatment of PI3K-addicted tumors. These recent developments call for a re-evaluation of the oncogenic mechanisms behind PI3K pathway alterations. This pathway is unique in that every major node is frequently mutated or amplified in a wide variety of solid tumors. Receptor tyrosine kinases upstream of PI3K, the p110 alpha catalytic subunit of PI3K, the downstream kinase, AKT, and the negative regulator, PTEN, are all frequently altered in cancer. In this review, we will examine the oncogenic properties of these genetic alterations to understand whether they are redundant or distinct and propose treatment strategies tailored for these genetic lesions.
Figures
Figure 1
The PI3K signaling axis. Activation of RTKs recruits PI3K directly or through adaptor proteins such as the GAB proteins. PI3K phosphorylates PIP2 to generate PIP3, which leads to AKT activation and activation of numerous effectors that regulate critical cellular functions in cancer cells. PTEN negatively regulates this process through dephosphorylation of PIP3. All major members of this signaling axis are frequently altered in cancer. PI3K, phosphoinositide 3-kinase; RTKs, receptor tyrosine kinases.
Figure 2
Possible models of oncogenicity. In model 1, genetic alterations in any of the PI3K pathway members lead to amplification of PI3K signaling. In model 2, alterations of RTKs, RAS or PTEN in addition to mutant PIK3CA lead to activation of non-overlapping pathways or disrupt negative feedback loops to enhance PI3K signaling. In model 3, oncogenic RAS leads to tumorigenesis via a mechanism independent of PI3K. In model 4, loss of PTEN leads to p53-dependent cellular senescence, which may be overcome by acquisition of an additional mutation in the pathway. In model 5, activation of PI3K is enhanced through interactions with oncogenic RAS. PI3K, phosphoinositide 3-kinase; RTKs, receptor tyrosine kinases.
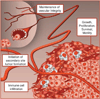
Figure 3
PI3K in the tumor microenvironment. In tumor cells, PI3K plays an important role in tumor initiation, growth and proliferation. PI3K also plays a major role in endothelial and immune cells that support tumor growth, intravasation and invasion. Inhibition of PI3K may thus have the potential to inhibit formation of secondary-site metastases. PI3K, phosphoinositide 3-kinase.
Similar articles
- Class I PI3K in oncogenic cellular transformation.
Zhao L, Vogt PK. Zhao L, et al. Oncogene. 2008 Sep 18;27(41):5486-96. doi: 10.1038/onc.2008.244. Oncogene. 2008. PMID: 18794883 Free PMC article. Review. - PIK3CA cooperates with other phosphatidylinositol 3'-kinase pathway mutations to effect oncogenic transformation.
Oda K, Okada J, Timmerman L, Rodriguez-Viciana P, Stokoe D, Shoji K, Taketani Y, Kuramoto H, Knight ZA, Shokat KM, McCormick F. Oda K, et al. Cancer Res. 2008 Oct 1;68(19):8127-36. doi: 10.1158/0008-5472.CAN-08-0755. Cancer Res. 2008. PMID: 18829572 - The relation between PI3K/AKT signalling pathway and cancer.
Noorolyai S, Shajari N, Baghbani E, Sadreddini S, Baradaran B. Noorolyai S, et al. Gene. 2019 May 25;698:120-128. doi: 10.1016/j.gene.2019.02.076. Epub 2019 Mar 5. Gene. 2019. PMID: 30849534 Review. - Phosphatidylinositol 3-kinase pathway genomic alterations in 60,991 diverse solid tumors informs targeted therapy opportunities.
Millis SZ, Jardim DL, Albacker L, Ross JS, Miller VA, Ali SM, Kurzrock R. Millis SZ, et al. Cancer. 2019 Apr 1;125(7):1185-1199. doi: 10.1002/cncr.31921. Epub 2018 Dec 24. Cancer. 2019. PMID: 30582752 Free PMC article. - Frequent PTEN loss and differential HER2/PI3K signaling pathway alterations in salivary duct carcinoma: Implications for targeted therapy.
Saintigny P, Mitani Y, Pytynia KB, Ferrarotto R, Roberts DB, Weber RS, Kies MS, Maity SN, Lin SH, El-Naggar AK. Saintigny P, et al. Cancer. 2018 Sep 15;124(18):3693-3705. doi: 10.1002/cncr.31600. Epub 2018 Oct 5. Cancer. 2018. PMID: 30289966 Free PMC article.
Cited by
- Targeted nanoconjugate co-delivering siRNA and tyrosine kinase inhibitor to KRAS mutant NSCLC dissociates GAB1-SHP2 post oncogene knockdown.
Srikar R, Suresh D, Zambre A, Taylor K, Chapman S, Leevy M, Upendran A, Kannan R. Srikar R, et al. Sci Rep. 2016 Aug 17;6:30245. doi: 10.1038/srep30245. Sci Rep. 2016. PMID: 27530552 Free PMC article. - Translational Advances in the Field of Pulmonary Hypertension. From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs.
Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Pullamsetti SS, et al. Am J Respir Crit Care Med. 2017 Feb 15;195(4):425-437. doi: 10.1164/rccm.201606-1226PP. Am J Respir Crit Care Med. 2017. PMID: 27627135 Free PMC article. Review. No abstract available. - Downregulation of cancer stem cell properties via mTOR signaling pathway inhibition by rapamycin in nasopharyngeal carcinoma.
Yang C, Zhang Y, Zhang Y, Zhang Z, Peng J, Li Z, Han L, You Q, Chen X, Rao X, Zhu Y, Liao Z. Yang C, et al. Int J Oncol. 2015 Sep;47(3):909-17. doi: 10.3892/ijo.2015.3100. Epub 2015 Jul 21. Int J Oncol. 2015. PMID: 26202311 Free PMC article. - Emerging therapeutics targeting mRNA translation.
Malina A, Mills JR, Pelletier J. Malina A, et al. Cold Spring Harb Perspect Biol. 2012 Apr 1;4(4):a012377. doi: 10.1101/cshperspect.a012377. Cold Spring Harb Perspect Biol. 2012. PMID: 22474009 Free PMC article. - The interferon-inducible protein viperin controls cancer metabolic reprogramming to enhance cancer progression.
Choi KM, Kim JJ, Yoo J, Kim KS, Gu Y, Eom J, Jeong H, Kim K, Nam KT, Park YS, Chung JY, Seo JY. Choi KM, et al. J Clin Invest. 2022 Dec 15;132(24):e157302. doi: 10.1172/JCI157302. J Clin Invest. 2022. PMID: 36227691 Free PMC article.
References
- Abubaker J, Bavi P, Al-Harbi S, Ibrahim M, Siraj AK, Al-Sanea N, et al. Clinicopathological analysis of colorectal cancers with PIK3CA mutations in Middle Eastern population. Oncogene. 2008;27:3539–3545. - PubMed
- Arteaga CL. EGF receptor mutations in lung cancer: from humans to mice and maybe back to humans. Cancer Cell. 2006;9:421–423. - PubMed
- Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. - PubMed
- Barault L, Veyrie N, Jooste V, Lecorre D, Chapusot C, Ferraz JM, et al. Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH kinase) signaling network correlate with poor survival in a population-based series of colon cancers. Int J Cancer. 2008;122:2255–2259. - PubMed
- Barnes KR, Blois J, Smith A, Yuan H, Reynolds F, Weissleder R, et al. Fate of a bioactive fluorescent wortmannin derivative in cells. Bioconjug Chem. 2008;19:130–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous