Glutathione: overview of its protective roles, measurement, and biosynthesis - PubMed (original) (raw)
Review
Glutathione: overview of its protective roles, measurement, and biosynthesis
Henry Jay Forman et al. Mol Aspects Med. 2009 Feb-Apr.
Abstract
This review is the introduction to a special issue concerning, glutathione (GSH), the most abundant low molecular weight thiol compound synthesized in cells. GSH plays critical roles in protecting cells from oxidative damage and the toxicity of xenobiotic electrophiles, and maintaining redox homeostasis. Here, the functions and GSH and the sources of oxidants and electrophiles, the elimination of oxidants by reduction and electrophiles by conjugation with GSH are briefly described. Methods of assessing GSH status in the cells are also described. GSH synthesis and its regulation are addressed along with therapeutic approaches for manipulating GSH content that have been proposed. The purpose here is to provide a brief overview of some of the important aspects of glutathione metabolism as part of this special issue that will provide a more comprehensive review of the state of knowledge regarding this essential molecule.
Figures
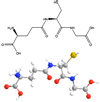
Fig. 1
Glutathione structure. A stereochemical and ball and stick figure showing γ-glutamyl-cysteinyl-glycine are shown.
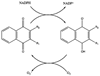
Fig. 2
Redox cycling of 1,4-naphthoquinones. A naphthoquinone with two variable groups (R) can be reduced by NADPH (or NADH, which is not shown) enzymatically to the semiquinone radical and then will react with oxygen to generate superoxide and restore the naphthoquinone.
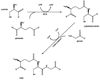
Fig. 3
Reactions of glutathione with hypochlorous acid. GSH and HOCl can react to produce several different products.
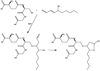
Fig. 4
Formation of protein mixed disulfide. Both glutathione peroxidases and peroxiredoxin 6 can catalyze the oxidation of glutathione by hydrogen peroxide to glutathione disulfide and water. GSSG can then undergo an exchange reaction with protein sulfhydryl to form PSSG, which is usually catalyzed by a protein disulfide isomerase. An alternative mechanism is the oxidation of a protein thiolate to a sulfenic acid, which then will react with GSH to form PSSG and water.
Fig. 5
Glutathione conjugations with 4-hydroxynonenal. Glutathione S-transferases catalyze the conjugation of GSH with HNE. This is a Michael addition that can slowly occur non-enzymatically.
Fig. 6
Measurements of thiols. (a) Reaction of GSH with DTNB produces an adduct and TNB, which is measured spectrofluorometrically or spectrophometrically; (b) total glutathione can be determined by recycling of GSSG produced in the reaction in (a) and measuring the rate of TNB; (c) Glutathione and related compounds are first derivatized with iodoacetate followed by a second derivatization with 1-fluoro-2,4-dinitrophenol. The second products are then separated by HPLC and measured spectrofluorometrically; (d) Reaction of glutathione with orthophthaldehyde (OPT) yields a product that can be measured spectrofluorometrically.
Fig. 6
Measurements of thiols. (a) Reaction of GSH with DTNB produces an adduct and TNB, which is measured spectrofluorometrically or spectrophometrically; (b) total glutathione can be determined by recycling of GSSG produced in the reaction in (a) and measuring the rate of TNB; (c) Glutathione and related compounds are first derivatized with iodoacetate followed by a second derivatization with 1-fluoro-2,4-dinitrophenol. The second products are then separated by HPLC and measured spectrofluorometrically; (d) Reaction of glutathione with orthophthaldehyde (OPT) yields a product that can be measured spectrofluorometrically.
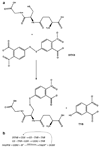
Fig. 7
Glutathione synthesis. The sequential ATP dependent formation of amide bonds between cysteine and the γ-carboxyl group of glutamate and then between glycine and cysteine are shown.
Similar articles
- Assessment of glutathione homeostasis.
Giordano G, White CC, Costa LG. Giordano G, et al. Methods Mol Biol. 2011;758:205-14. doi: 10.1007/978-1-61779-170-3_14. Methods Mol Biol. 2011. PMID: 21815068 - Glutathione synthesis and its role in redox signaling.
Zhang H, Forman HJ. Zhang H, et al. Semin Cell Dev Biol. 2012 Sep;23(7):722-8. doi: 10.1016/j.semcdb.2012.03.017. Epub 2012 Apr 3. Semin Cell Dev Biol. 2012. PMID: 22504020 Free PMC article. Review. - Glutathione and glutathione-dependent enzymes: From biochemistry to gerontology and successful aging.
Lapenna D. Lapenna D. Ageing Res Rev. 2023 Dec;92:102066. doi: 10.1016/j.arr.2023.102066. Epub 2023 Sep 7. Ageing Res Rev. 2023. PMID: 37683986 Review. - Glutathione synthesis.
Lu SC. Lu SC. Biochim Biophys Acta. 2013 May;1830(5):3143-53. doi: 10.1016/j.bbagen.2012.09.008. Epub 2012 Sep 17. Biochim Biophys Acta. 2013. PMID: 22995213 Free PMC article. Review. - Biologic and pharmacologic regulation of mammalian glutathione synthesis.
Griffith OW. Griffith OW. Free Radic Biol Med. 1999 Nov;27(9-10):922-35. doi: 10.1016/s0891-5849(99)00176-8. Free Radic Biol Med. 1999. PMID: 10569625 Review.
Cited by
- Responses in weanling pigs fed low protein diets supplemented with dietary nucleotides.
Lawal AS, Ogunribido TZ, Fu Y, Adeola O, Ajuwon KM. Lawal AS, et al. Transl Anim Sci. 2024 Oct 3;8:txae142. doi: 10.1093/tas/txae142. eCollection 2024. Transl Anim Sci. 2024. PMID: 39444714 Free PMC article. - Glutaminolysis dynamics during astrocytoma progression correlates with tumor aggressiveness.
Moreira Franco YE, Alves MJ, Uno M, Moretti IF, Trombetta-Lima M, de Siqueira Santos S, Dos Santos AF, Arini GS, Baptista MS, Lerario AM, Oba-Shinjo SM, Marie SKN. Moreira Franco YE, et al. Cancer Metab. 2021 Apr 28;9(1):18. doi: 10.1186/s40170-021-00255-8. Cancer Metab. 2021. PMID: 33910646 Free PMC article. - Antibiotics-free compounds for managing carbapenem-resistant bacteria; a narrative review.
Shariati A, Kashi M, Chegini Z, Hosseini SM. Shariati A, et al. Front Pharmacol. 2024 Sep 17;15:1467086. doi: 10.3389/fphar.2024.1467086. eCollection 2024. Front Pharmacol. 2024. PMID: 39355778 Free PMC article. Review. - Biochemical Control of the Mitochondrial Protein MitoNEET by Biological Thiols and Lipid-derived Electrophiles.
Skolik RA, Geldenhuys WJ, Konkle ME, Menze MA. Skolik RA, et al. Adv Redox Res. 2023 Apr;7:100059. doi: 10.1016/j.arres.2022.100059. Epub 2022 Dec 23. Adv Redox Res. 2023. PMID: 39364216 Free PMC article. - GSH and Ferroptosis: Side-by-Side Partners in the Fight against Tumors.
Jiang Y, Glandorff C, Sun M. Jiang Y, et al. Antioxidants (Basel). 2024 Jun 6;13(6):697. doi: 10.3390/antiox13060697. Antioxidants (Basel). 2024. PMID: 38929136 Free PMC article. Review.
References
- Akerboom TPM, Sies H. Assay of glutathione, glutathione disulfide, and glutathione mixed disulfides in biological samples. Methods Enzymol. 1981;77:373–382. - PubMed
- Alary J, Gueraud F, et al. Fate of 4-hydroxynonenal in vivo: disposition and metabolic pathways. Mol. Aspects Med. 2003;24(4–5):177–187. - PubMed
- Anderson ME, Bridges RJ, et al. Direct evidence for inter-organ transport of glutathione and that the non-filtration renal mechanism for glutathione utilization involves g-glutamyl transpeptidase. Biochem. Biophys. Res. Commun. 1980;96(2):848–853. - PubMed
- Anderson CP, Tsai J, et al. Buthionine sulphoximine alone and in combination with melphalan (L-PAM) is highly cytotoxic for human neuroblastoma cell lines. Eur. J. Cancer. 1997;33(12):2016–2019. - PubMed
- Bakkenist AR, de Boer JE, et al. The halide complexes of myeloperoxidase and the mechanism of the halogenation reactions. Biochim. Biophys. Acta. 1980;613(2):337–348. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources