Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites - PubMed (original) (raw)
Review
Characterisation of effector mechanisms at the host:parasite interface during the immune response to tissue-dwelling intestinal nematode parasites
Nirav Patel et al. Int J Parasitol. 2009 Jan.
Abstract
The protective immune response that develops following infection with many tissue-dwelling intestinal nematode parasites is characterised by elevations in IL-4 and IL-13 and increased numbers of CD4+ T cells, granulocytes and macrophages. These cells accumulate at the site of infection and in many cases can mediate resistance to these large multicellular pathogens. Recent studies suggest novel potential mechanisms mediated by these immune cell populations through their differential activation and ability to stimulate production of novel effector molecules. These newly discovered protective mechanisms may provide novel strategies to develop immunotherapies and vaccines against this group of pathogens. In this review, we will examine recent studies elucidating mechanisms of host protection against three widely-used experimental murine models of tissue-dwelling intestinal nematode parasites: Heligmosomoides polygyrus, Trichuris muris and Trichinella spiralis.
Figures
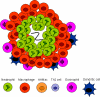
Fig. 1
Th1- and Th2-type granulomas have distinct cell types and phenotypes. (A) At day 4 after primary inoculation with Heligmosomoides polygyrus, the tissue-dwelling larva is surrounded by a neutrophilic infiltrate and macrophages. Th2 cells are not found at this early timepoint. (B) At day 4 after secondary challenge, the H. polygyrus larva provokes a Th2-type granuloma, characterized by Th2 cells, more eosinophils, and alternatively activated macrophages (AAMacs). (C) The response to Mycobacterium tuberculosis is typical of a Th1-type granuloma. Th1-derived IFN-γ results in classically activated macrophages, which use inducible nitric oxide synthase (iNOS) to generate microbicidal products that destroy phagocytosed bacteria.
Fig. 1
Th1- and Th2-type granulomas have distinct cell types and phenotypes. (A) At day 4 after primary inoculation with Heligmosomoides polygyrus, the tissue-dwelling larva is surrounded by a neutrophilic infiltrate and macrophages. Th2 cells are not found at this early timepoint. (B) At day 4 after secondary challenge, the H. polygyrus larva provokes a Th2-type granuloma, characterized by Th2 cells, more eosinophils, and alternatively activated macrophages (AAMacs). (C) The response to Mycobacterium tuberculosis is typical of a Th1-type granuloma. Th1-derived IFN-γ results in classically activated macrophages, which use inducible nitric oxide synthase (iNOS) to generate microbicidal products that destroy phagocytosed bacteria.
Fig. 1
Th1- and Th2-type granulomas have distinct cell types and phenotypes. (A) At day 4 after primary inoculation with Heligmosomoides polygyrus, the tissue-dwelling larva is surrounded by a neutrophilic infiltrate and macrophages. Th2 cells are not found at this early timepoint. (B) At day 4 after secondary challenge, the H. polygyrus larva provokes a Th2-type granuloma, characterized by Th2 cells, more eosinophils, and alternatively activated macrophages (AAMacs). (C) The response to Mycobacterium tuberculosis is typical of a Th1-type granuloma. Th1-derived IFN-γ results in classically activated macrophages, which use inducible nitric oxide synthase (iNOS) to generate microbicidal products that destroy phagocytosed bacteria.
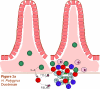
Fig. 2
Different components of the Th2-type response are effective against different helminthic parasites. Responses involve Th2 cells (dark green), neutrophils (light blue), macrophages (dark blue), alternatively activated macrophages (AAMacs; red), eosinophils (purple), goblet cells (light green), epithelial cells (pink), epithelial syncytium (maroon), mast cells (orange) and secreted factors. (A) The localized memory response to the Heligmosomoides polygyrus tissue-dwelling larva is characterized by AAMacs. AAMac-mediated protection is arginase-dependent and includes secretion of the chitinase-like protein Ym-1 and resistin-like molecule α (RELMα). (B) An IL-13-dependent “epithelial escalator” uses increased cell turnover to displace the burrowing head of Trichuris muris. Epithelial cell thymic stromal lymphopoietin (TSLP) may stimulate Th2 responses and inhibit Th1-type inflammatory responses, and goblet cell resistin-like molecule β (RELMβ) may bind the worm. Eosinophils accumulate in the lamina propria but are not required for parasite expulsion. (C) Th2 cytokines induce mast cell protease 1 (mMCP-1) which disrupts epithelial cell tight junctions, and goblet cell hyperplasia, together creating a “leaky” gut environment that favors expulsion of Trichinella spiralis from the syncytium of epithelial cells where it rapidly matures and reproduces.
Fig. 2
Different components of the Th2-type response are effective against different helminthic parasites. Responses involve Th2 cells (dark green), neutrophils (light blue), macrophages (dark blue), alternatively activated macrophages (AAMacs; red), eosinophils (purple), goblet cells (light green), epithelial cells (pink), epithelial syncytium (maroon), mast cells (orange) and secreted factors. (A) The localized memory response to the Heligmosomoides polygyrus tissue-dwelling larva is characterized by AAMacs. AAMac-mediated protection is arginase-dependent and includes secretion of the chitinase-like protein Ym-1 and resistin-like molecule α (RELMα). (B) An IL-13-dependent “epithelial escalator” uses increased cell turnover to displace the burrowing head of Trichuris muris. Epithelial cell thymic stromal lymphopoietin (TSLP) may stimulate Th2 responses and inhibit Th1-type inflammatory responses, and goblet cell resistin-like molecule β (RELMβ) may bind the worm. Eosinophils accumulate in the lamina propria but are not required for parasite expulsion. (C) Th2 cytokines induce mast cell protease 1 (mMCP-1) which disrupts epithelial cell tight junctions, and goblet cell hyperplasia, together creating a “leaky” gut environment that favors expulsion of Trichinella spiralis from the syncytium of epithelial cells where it rapidly matures and reproduces.
Fig. 2
Different components of the Th2-type response are effective against different helminthic parasites. Responses involve Th2 cells (dark green), neutrophils (light blue), macrophages (dark blue), alternatively activated macrophages (AAMacs; red), eosinophils (purple), goblet cells (light green), epithelial cells (pink), epithelial syncytium (maroon), mast cells (orange) and secreted factors. (A) The localized memory response to the Heligmosomoides polygyrus tissue-dwelling larva is characterized by AAMacs. AAMac-mediated protection is arginase-dependent and includes secretion of the chitinase-like protein Ym-1 and resistin-like molecule α (RELMα). (B) An IL-13-dependent “epithelial escalator” uses increased cell turnover to displace the burrowing head of Trichuris muris. Epithelial cell thymic stromal lymphopoietin (TSLP) may stimulate Th2 responses and inhibit Th1-type inflammatory responses, and goblet cell resistin-like molecule β (RELMβ) may bind the worm. Eosinophils accumulate in the lamina propria but are not required for parasite expulsion. (C) Th2 cytokines induce mast cell protease 1 (mMCP-1) which disrupts epithelial cell tight junctions, and goblet cell hyperplasia, together creating a “leaky” gut environment that favors expulsion of Trichinella spiralis from the syncytium of epithelial cells where it rapidly matures and reproduces.
Similar articles
- [Regulation of the immune response to intestinal nematode infections in mice].
Cywińska A, Schollenberger A. Cywińska A, et al. Wiad Parazytol. 2000;46(4):421-31. Wiad Parazytol. 2000. PMID: 16886322 Review. Polish. - [Changes in the immune response of BALB/c mice coinfected with Heligmosomoides polygyrus and Trichinella spiralis].
Doligalska M, Rzepecka J, Moskwa B, Laskowska M. Doligalska M, et al. Wiad Parazytol. 2001;47(4):735-9. Wiad Parazytol. 2001. PMID: 16886419 Polish. - Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites.
Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF Jr. Finkelman FD, et al. Immunol Rev. 2004 Oct;201:139-55. doi: 10.1111/j.0105-2896.2004.00192.x. Immunol Rev. 2004. PMID: 15361238 Review. - Memory Th2 effector cells can develop in the absence of B7-1/B7-2, CD28 interactions, and effector Th cells after priming with an intestinal nematode parasite.
Ekkens MJ, Liu Z, Liu Q, Foster A, Whitmire J, Pesce J, Sharpe AH, Urban JF, Gause WC. Ekkens MJ, et al. J Immunol. 2002 Jun 15;168(12):6344-51. doi: 10.4049/jimmunol.168.12.6344. J Immunol. 2002. PMID: 12055251 - Contrasting effects of acute and chronic gastro-intestinal helminth infections on a heterologous immune response in a transgenic adoptive transfer model.
Boitelle A, Di Lorenzo C, Scales HE, Devaney E, Kennedy MW, Garside P, Lawrence CE. Boitelle A, et al. Int J Parasitol. 2005 Jun;35(7):765-75. doi: 10.1016/j.ijpara.2005.02.013. Epub 2005 Apr 18. Int J Parasitol. 2005. PMID: 15893319
Cited by
- The role of IL-33/ST2, IL-4, and eosinophils on the airway hyperresponsiveness induced by Strongyloides venezuelensis in BALB/c mice.
Araujo ES, de Jesus Pereira CA, de Moura Pereira AT, Moreira JM, de Rezende MC, Rodrigues JL, Teixeira MM, Negrão-Corrêa D. Araujo ES, et al. Parasitol Res. 2016 Aug;115(8):3107-17. doi: 10.1007/s00436-016-5066-6. Epub 2016 Apr 22. Parasitol Res. 2016. PMID: 27102638 - Th2/1 Hybrid Cells Occurring in Murine and Human Strongyloidiasis Share Effector Functions of Th1 Cells.
Bock CN, Babu S, Breloer M, Rajamanickam A, Boothra Y, Brunn ML, Kühl AA, Merle R, Löhning M, Hartmann S, Rausch S. Bock CN, et al. Front Cell Infect Microbiol. 2017 Jun 20;7:261. doi: 10.3389/fcimb.2017.00261. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28676845 Free PMC article. - B cells have distinct roles in host protection against different nematode parasites.
Liu Q, Kreider T, Bowdridge S, Liu Z, Song Y, Gaydo AG, Urban JF Jr, Gause WC. Liu Q, et al. J Immunol. 2010 May 1;184(9):5213-23. doi: 10.4049/jimmunol.0902879. Epub 2010 Mar 31. J Immunol. 2010. PMID: 20357259 Free PMC article. - A role for IL-22 in the relationship between intestinal helminths, gut microbiota and mucosal immunity.
Leung JM, Loke P. Leung JM, et al. Int J Parasitol. 2013 Mar;43(3-4):253-7. doi: 10.1016/j.ijpara.2012.10.015. Epub 2012 Nov 20. Int J Parasitol. 2013. PMID: 23178750 Free PMC article. Review. - Sexual Dimorphism of the Neuroimmunoendocrine Response in the Spleen during a Helminth Infection: A New Role for an Old Player?
Nava-Castro KE, Pavón L, Becerril-Villanueva LE, Ponce-Regalado MD, Aguilar-Díaz H, Segovia-Mendoza M, Morales-Montor J. Nava-Castro KE, et al. Pathogens. 2022 Mar 1;11(3):308. doi: 10.3390/pathogens11030308. Pathogens. 2022. PMID: 35335632 Free PMC article.
References
- Al-Qaoud KM, Pearlman E, Hartung T, Klukowski J, Fleischer B, Hoerauf A. A new mechanism for IL-5-dependent helminth control: neutrophil accumulation and neutrophil-mediated worm encapsulation in murine filariasis are abolished in the absence of IL-5. Int Immunol. 2000;12:899–908. - PubMed
- Alizadeh H, Murrell KD. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. J Parasitol. 1984;70:767–773. - PubMed
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI031678/AI/NIAID NIH HHS/United States
- R01 AI066188-04/AI/NIAID NIH HHS/United States
- R01 AI031678-12/AI/NIAID NIH HHS/United States
- R01 AI066188-05/AI/NIAID NIH HHS/United States
- R01 AI031678-13/AI/NIAID NIH HHS/United States
- R01 AI066188-03/AI/NIAID NIH HHS/United States
- R01 AI031678-14/AI/NIAID NIH HHS/United States
- R01 AI066188/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials