A self-associating protein critical for chromosome attachment, division, and polar organization in caulobacter - PubMed (original) (raw)
A self-associating protein critical for chromosome attachment, division, and polar organization in caulobacter
Gitte Ebersbach et al. Cell. 2008.
Abstract
Cell polarization is an integral part of many unrelated bacterial processes. How intrinsic cell polarization is achieved is poorly understood. Here, we provide evidence that Caulobacter crescentus uses a multimeric pole-organizing factor (PopZ) that serves as a hub to concurrently achieve several polarizing functions. During chromosome segregation, polar PopZ captures the ParB*ori complex and thereby anchors sister chromosomes at opposite poles. This step is essential for stabilizing bipolar gradients of a cell division inhibitor and setting up division near midcell. PopZ also affects polar stalk morphogenesis and mediates the polar localization of the morphogenetic and cell cycle signaling proteins CckA and DivJ. Polar accumulation of PopZ, which is central to its polarizing activity, can be achieved independently of division and does not appear to be dictated by the pole curvature. Instead, evidence suggests that localization of PopZ largely relies on PopZ multimerization in chromosome-free regions, consistent with a self-organizing mechanism.
Figures
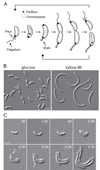
Figure 1. A multicopy plasmid screen identifies a factor involved in cell division placement
(A) C. crescentus cell cycle schematic showing several cell polarization events. Pili, stalk and flagellum form at a defined pole, and segregation of the chromosomal origins of replication bound to ParB (ParB•ori) results in ParB•ori complexes at opposite poles. (B) DIC microscopy images of CB15N/pJS14Pxyl-PopZ cells growing in the presence of glucose (no induction) or presence of xylose (which causes popZ overexpression and cell filamentation). (C) Time-lapse DIC microscopy of strain CB15N/pJS14Pxyl-PopZ. The slide contained xylose to induce PopZ overproduction. Arrows indicate formation of minicells. Scale bars, 2µm.
Figure 2. PopZ colocalizes with ParB at the poles
(A) Time-lapse microscopy of synchronized cells producing PopZ-YFP (CB15N popZ::pBGent-PopZ-YFP). Numbers indicate h and min. (B) Time-lapse microscopy of CB15N parB::cfp-parB popZ::pBGent-PopZ-YFP cells shows that PopZ-YFP and CFP-ParB bound to ori have similar polar localization patterns. The second frame shows one of the duplicated CFP-ParB•ori migrating toward the new pole. Scale bar, 2µm.
Figure 3. Deletion of PopZ results in minicelling, cell filamentation and failure of polar chromosome attachment
(A) Time-lapse microscopy of strain CB15N Δ_popZ_ grown in PYE. Arrows show formation of minicells. Numbers indicate h and min. (B) Localization of CFP-ParB (red overlaid with DIC) in wild-type, Δ_popZ_ and FtsZ-depleted cells. (C) Time-lapse images showing CFP-ParB localization (red) in a wild-type background (CB15N parB::cfp-parB). (D) Time-lapse images (see Movie 2) showing CFP-ParB localization in Δ_popZ_ cells (CB15N Δ_popZ parB::cfp-parB_). (E) Kymograph analysis of CFP-ParB movement in Δ_popZ_ cells from a time-lapse recording (Movie 3). On the left is an overlay of CFP-ParB fluorescence (red) and DIC images, corresponding to the first time point of the time-lapse sequence. On the right is kymographs showing the movement of two of the CFP-ParB foci (one polar and one internal) over time within defined regions (indicated by dotted lines “a” and “b” in the overlay) (F) Same as (E) for FtsZ-depleted cells (see Movie 4). While the internal CFP-ParB focus shows motion, the two polar foci display little, if any.. (G) Bipolar localization of PopZ-YFP in FtsZ-depleted cells. (H) PopZ-dependent attachment of CFP-ParB•ori at poles. Strain CB15N Δ_popZ xylX::pXmYFP4-PopZ parB::cfp-parB_ was grown in PYE with glucose under which conditions mYFP-PopZ (only source of PopZ) was depleted for 20h. These cells were then placed on an M2G agarose pad containing xylose in order to restore mYFP-PopZ synthesis. Time-lapse imaging shows that emerging mYFP-PopZ foci (red) capture CFP-ParB•ori foci (green). Yellow arrows indicate overlapping mYFP-PopZ and CFP-ParB foci. (I) Immunoblotting of co-IP eluates from lysates of CB15N popZ::pBGent-PopZ-YFP cells and wild-type CB15N cells (negative control). The amount of cell lysate loaded (two lanes to the right) was 1/100 of the amount used for the immunoprecipitates. (J) Purified His6-ParB and His6-PopZ proteins co-immunoprecipitate. The presence of His6-ParB and His6-PopZ in the eluate was determined by immunoblotting using α-Tetra-His antibodies. Controls lacking either His6-PopZ or His6-ParB were included. To identify the position of each protein after SDS-PAGE, samples of His6-ParB and His6-PopZ were loaded directly onto the gel (two lanes to the right, 25ng).
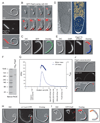
Figure 4. PopZ multimerizes and during overproduction, it forms a large polar accumulation that excludes DNA and ribosomes
(A) Microscopy images of strain CB15N/pJS14Pxyl-PopZ-TC grown with xylose for 6h to achieve overproduction of PopZ-TC. (B) Time-lapse microscopy of strain CB15N/pJS14Pxyl-GFP-PopZ inducing GFP-PopZ (red) overproduction on the slide. (C) Microscopy images showing uniform GFP localization in a strain (CB15N xylX::pXGFP4/pJS14Pvan-PopZ) overproducing untagged PopZ. Accumulation of PopZ during overproduction creates a shading difference in DIC images (marked by arrows and broken line). (D) Electron cryo-tomogram of CB15N/pJS14Pxyl-PopZ grown in the presence of xylose for 6.5h to overproduce PopZ. Left image: 15 nm thick slice from the median filtered tomogram. Right image: 58 nm thick section of the segmentation including the region shown on the left. SL, surface layer; OM, outer membrane; IM, inner membrane; Rib, probable ribosome (yellow in right panel). Scale bar, 100nm. (E) Microscopy images showing FlAsH-stained PopZ-TC-rich region of a CB15N/pJS14Pxyl-PopZ-TC cell grown in the presence of xylose for 6h. DNA was stained with DAPI. Broken line and arrows indicate the PopZ-rich region. (F) Purified His6-PopZ forms a large complex. The protein (1 µg) was loaded onto a 4–15% gradient native gel (Bio-Rad) together with a protein ladder. (G) Determination of MW of His6-PopZ by using SEC-UV/LS/RI. The elution profile of His6-PopZ is shown as a blue continuous line. The clustered points represent light scattering data converted to MW. His6-PopZ MW showed a range from 125 to 175 kDa, with an average MW of 157 kDa, indicating that PopZ forms a 6–8mer in solution under these conditions. Vertical arrows at the bottom indicate the column void volume and the elution volume of 4 standards: Trypsin Inhibitor (20 kDa), Bovine Serum Albumin (66 kDa), Beta Amylase (220 kDa) and Apo Ferritin (475 kDa). (H) Ori is located at the border of the PopZ-rich region in _popZ_-overexpressing cells. Strain CB15N Cori::Cori-lacOp-kan xylX::pHPV472/pJS14Pvan-PopZ was grown in PYE and popZ overexpression (under the vanillate-inducible promoter) was induced with 0.5 mM vanillic acid for 8h. The location of ori was visualized via LacI-CFP bound to an array of lac operator sites inserted near ori. Synthesis of LacI-CFP (under xylose inducible expression) was achieved by addition of 2 mM xylose for 1h prior to visualization. At the time of xylose addition, 50 µM IPTG was added in order to decrease potential side effects originating from binding of LacI-CFP to its operator sites (Viollier et al., 2004). Broken line and arrows indicate the PopZ-rich region. (I) CFP-ParB localizes to the PopZ-rich region of cells (CB15N parB:: cfp-parB/pJS14Pxyl-PopZ) overproducing untagged PopZ for 8h. Broken line and arrows indicate the PopZ-rich region. Red arrows indicate CFP-ParB foci at the border of the PopZ region and along the cell length. (J) MipZ-YFP localizes at the border of the PopZ-rich region and occasionally along the cell filament in PopZ-overproducing cells (CB15N mipZ::mipZ-YFP/pJS14Pxyl-PopZ). Broken line and arrows indicate the PopZ-rich region. Scale bars, 2µm except for (D).
Figure 5. PopZ affects the localization of CckA, DivJ and TipN
(A) Microscopy images showing the localization of chromosome-encoded fusion proteins DivJ-YFP or CckA-mGFP in wild-type and Δ_popZ_ cells. (B) PopZ-YFP was immunoprecipitated from cell lysates of strains CB15N popZ:: pBGent-PopZ-YFP and CB15N (negative control). PopZ-YFP IP and co-IP of crescentin (negative control), DivJ or CckA were analyzed by performing immunoblotting on the same membrane. The amount of cell lysate loaded (two lanes to the right) was 1/100 of the amount used for the immunoprecipitates. (C) Microscopy images showing the localization of DivJ-YFP or CckA-mGFP in cells overproducing PopZ from plasmid pJS14Pxyl-PopZ. PopZ-rich region is show by broken line. (D) Microscopy images from a time-lapse sequence showing the localization of chromosome-encoded TipN-GFP in Δ_popZ_ cells during growth. (E) Depletion of TipN in a Δ_popZ_ background results in a severe synthetic division phenotype. Micrographs of strains CB15N Δ_tipN xylX::Pxyl-tipN_ and CB15N Δ_popZ_ Δ_tipN xylX::Pxyl-tipN_ grown in PYE-xylose (causing TipN synthesis) or PYE-glucose (causing TipN depletion) for 20h. Scale bars, 2 µm, except for (F).
Figure 6. PopZ localizes to the pole in E. coli
(A) Microscopy images showing polar localization of C. crescentus TC-tagged PopZ expressed in E. coli. Strain MC1000/pBAD33PopZ-TC was grown at 30°C. PopZ-TC expression was induced by addition of arabinose for 1h and was visualized by staining with FlAsH for 30min. (B) Microscopy images showing PopZ-dependent localization of C. crescentus ParB to the pole of E. coli cells. Strain MC1000/pNDM220CFP-ParB/pBAD33PopZ-TC was grown as described in (A). CFP-ParB was induced by addition of 1 mM IPTG for 4h. When indicated, PopZ-TC expression was induced by adding arabinose for 2h. PopZ-TC was stained with ReAsH for 30 min to allow simultaneous visualization with CFP-ParB. It should be noted that ReAsH only stained a small percentage of the cells. However, similar results were obtained with a strain expressing YFP-PopZ and CFP-ParB (not shown). (C) Microscopy images showing a uniform localization of C. crescentus MipZ (used here as a control) in E. coli cells synthesizing PopZ-TC stained with ReAsH. Strain MC1000/pNDM220CFP-MipZ/pBAD33PopZ-TC was grown as described in (A). The synthesis of CFP-MipZ and PopZ-TC were induced by addition of 1 mM IPTG for 4h and arabinose for 2h, respectively. Scale bar, 2µm. (D) Strain CB15N xylX:: pXGFP4-PopZ/pJS14TipN was grown in PYE with 0.03% xylose. PopZ was found at the poles, including branched poles created by TipN overproduction. (E) Microscopy images showing the localization of mYFP-PopZ in E. coli protoplasts. Strain MC1000/pBAD33mYFP-PopZ was grown in LB at 37°C. Expression of mYFP-PopZ was induced by addition of arabinose for 30 min. (F) Selected frames from a time-lapse fluorescence microscopy experiment showing accumulation of mYFP-PopZ in a spheroid A22-treated E. coli cell. Strain MC1000/pBAD33mYFP-PopZ was grown at 30°C. A22 was added for 4h before cells were mounted on a pad containing A22 and 0.2% arabinose to induce mYFP-PopZ synthesis. Arrows show the appearance of mYFP-PopZ foci. (G) Selected frames from a time-lapse fluorescence microscopy experiment showing accumulation of mYFP-PopZ in rod-shaped E. coli. Strain MC1000/pBAD33mYFP-PopZ was grown at 30°C. The slide contained 0.2% arabinose to induce mYFP-PopZ synthesis. Arrows show the appearance of mYFP-PopZ foci. (H) Microscopy images of cephalexin-treated E. coli cells showing the formation of mYFP-PopZ foci (red) in chromosome-free regions including between nucleoids (stained with DAPI; green). MC1000/pBAD33mYFP-PopZ cells were grown at 30°C and treated with cephalexin (2.5h) before adding arabinose (1h) to induce expression of myfp-popZ. (I) PopZ-YFP localizes to chromosome-free regions at the poles of C. crescentus CB15N popZ::pBGent-PopZ-YFP. The image shows an overlay of PopZ-YFP (red) and DNA (green). (J) PopZ forms multiple foci exclusively in chromosome-free regions in a C. crescentus parEts ftsAts double mutant grown at the restrictive temperature. CB15 divD308(Ts)::pDW110 (parEp) divE309(Ts) xylX::pXmYFP4-popZ cells were grown in PYE at 30°C and then shifted to 37°C for 6h. mYFP-PopZ synthesis (red) was induced with xylose (0.3%) 1h before microscopy. DAPI stains the DNA (green). (K) Images of CB15N parB::cfp-parB popZ::pBGent-PopZ-YFP cells grown in M2G medium show the overlap (yellow) between CFP-ParB signal (green) and the pole-distal tip of the PopZ-YFP signal (red), consistent with PopZ forming a matrix under physiological conditions. Scale bars, 2 µm.
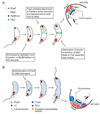
Figure 7. Proposed models for PopZ function and localization
(A) Model by which PopZ mediates the attachment of ori at the poles and thereby regulates cell division. Before DNA replication is initiated, the chromosome is tethered at the old pole via an interaction between PopZ and ParB bound to the parS sequence nearby ori. ParB also associates with MipZ and this association maintains FtsZ at the opposite pole. After replication is initiated, one of the duplicated ParB•ori complexes migrates toward the new pole where PopZ accumulates. PopZ captures the migrating ParB•ori and the immobilization of ParB•ori at both poles via PopZ generates stable bipolar accumulation of MipZ activity, which is critical for stable assembly of the FtsZ cytokinetic ring near midcell. (B) Model by which PopZ achieves polar localization and perform several polarizing activities. The newborn swarmer cell inherits a PopZ self-assembled matrix at the old pole from its mother cell. Over time, PopZ also self-associates in the chromosome-free space at the new pole where multimerization into a matrix is favored. The PopZ matrix, which continues to grow in size, serves as an organizing platform for the attachment of the chromosome via a ParB interaction and for the (direct or indirect) recruitment of CckA and DivJ.
Comment in
- Grasping at origins.
Ramamurthi KS, Losick R. Ramamurthi KS, et al. Cell. 2008 Sep 19;134(6):916-8. doi: 10.1016/j.cell.2008.09.004. Cell. 2008. PMID: 18805084
Similar articles
- A polymeric protein anchors the chromosomal origin/ParB complex at a bacterial cell pole.
Bowman GR, Comolli LR, Zhu J, Eckart M, Koenig M, Downing KH, Moerner WE, Earnest T, Shapiro L. Bowman GR, et al. Cell. 2008 Sep 19;134(6):945-55. doi: 10.1016/j.cell.2008.07.015. Cell. 2008. PMID: 18805088 Free PMC article. - A Localized Complex of Two Protein Oligomers Controls the Orientation of Cell Polarity.
Perez AM, Mann TH, Lasker K, Ahrens DG, Eckart MR, Shapiro L. Perez AM, et al. mBio. 2017 Feb 28;8(1):e02238-16. doi: 10.1128/mBio.02238-16. mBio. 2017. PMID: 28246363 Free PMC article. - Polar Organizing Protein PopZ Is Required for Chromosome Segregation in Agrobacterium tumefaciens.
Ehrle HM, Guidry JT, Iacovetto R, Salisbury AK, Sandidge DJ, Bowman GR. Ehrle HM, et al. J Bacteriol. 2017 Aug 8;199(17):e00111-17. doi: 10.1128/JB.00111-17. Print 2017 Sep 1. J Bacteriol. 2017. PMID: 28630129 Free PMC article. - End-in-Sight: Cell Polarization by the Polygamic Organizer PopZ.
Bergé M, Viollier PH. Bergé M, et al. Trends Microbiol. 2018 Apr;26(4):363-375. doi: 10.1016/j.tim.2017.11.007. Epub 2017 Nov 29. Trends Microbiol. 2018. PMID: 29198650 Review. - Cell cycle regulation in Caulobacter: location, location, location.
Goley ED, Iniesta AA, Shapiro L. Goley ED, et al. J Cell Sci. 2007 Oct 15;120(Pt 20):3501-7. doi: 10.1242/jcs.005967. J Cell Sci. 2007. PMID: 17928306 Review.
Cited by
- Phospho-signaling couples polar asymmetry and proteolysis within a membraneless microdomain in Caulobacter crescentus.
Ahmed YM, Brown LM, Varga K, Bowman GR. Ahmed YM, et al. Nat Commun. 2024 Oct 28;15(1):9282. doi: 10.1038/s41467-024-53395-y. Nat Commun. 2024. PMID: 39468040 Free PMC article. - Biomolecular condensates as stress sensors and modulators of bacterial signaling.
Sasazawa M, Tomares DT, Childers WS, Saurabh S. Sasazawa M, et al. PLoS Pathog. 2024 Aug 15;20(8):e1012413. doi: 10.1371/journal.ppat.1012413. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39146259 Free PMC article. Review. - Lifecycle of a predatory bacterium vampirizing its prey through the cell envelope and S-layer.
Santin YG, Sogues A, Bourigault Y, Remaut HK, Laloux G. Santin YG, et al. Nat Commun. 2024 Apr 27;15(1):3590. doi: 10.1038/s41467-024-48042-5. Nat Commun. 2024. PMID: 38678033 Free PMC article. - Three factors ParA, TipN, and DnaA-mediated chromosome replication initiation are contributors of centromere segregation in Caulobacter crescentus.
Letzkus M, Trela C, Mera PE. Letzkus M, et al. Mol Biol Cell. 2024 May 1;35(5):ar68. doi: 10.1091/mbc.E23-12-0503. Epub 2024 Apr 3. Mol Biol Cell. 2024. PMID: 38568781 Free PMC article. - Distinct dynamics and proximity networks of hub proteins at the prey-invading cell pole in a predatory bacterium.
Remy O, Santin YG, Jonckheere V, Tesseur C, Kaljević J, Van Damme P, Laloux G. Remy O, et al. J Bacteriol. 2024 Apr 18;206(4):e0001424. doi: 10.1128/jb.00014-24. Epub 2024 Mar 12. J Bacteriol. 2024. PMID: 38470120 Free PMC article.
References
- Bardy SL, Maddock JR. Polar explorations Recent insights into the polarity of bacterial proteins. Curr Opin Microbiol. 2007;10:617–623. - PubMed
- Ben-Yehuda S, Rudner DZ, Losick R. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science. 2003;299:532–536. - PubMed
- Biondi EG, Reisinger SJ, Skerker JM, Arif M, Perchuk BS, Ryan KR, Laub MT. Regulation of the bacterial cell cycle by an integrated genetic circuit. Nature. 2006;444:899–904. - PubMed
- Briegel A, Dias DP, Li Z, Jensen RB, Frangakis AS, Jensen GJ. Multiple large filament bundles observed in Caulobacter crescentus by electron cryotomography. Mol Microbiol. 2006;62:5–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI067548/AI/NIAID NIH HHS/United States
- GM065835/GM/NIGMS NIH HHS/United States
- Howard Hughes Medical Institute/United States
- R01 AI067548/AI/NIAID NIH HHS/United States
- R01 GM065835/GM/NIGMS NIH HHS/United States
- R01 GM065835-05/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases