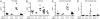Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger - PubMed (original) (raw)
. 2008 Sep 30;105(39):15028-33.
doi: 10.1073/pnas.0806619105. Epub 2008 Sep 19.
Brian R Lawson, Sophie Rutschmann, Michael Berger, Celine Eidenschenk, Amanda L Blasius, Eva Marie Y Moresco, Sosathya Sovath, Louise Cengia, Leonard D Shultz, Argyrios N Theofilopoulos, Sven Pettersson, Bruce Alan Beutler
Affiliations
- PMID: 18806225
- PMCID: PMC2567487
- DOI: 10.1073/pnas.0806619105
Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger
Ben A Croker et al. Proc Natl Acad Sci U S A. 2008.
Erratum in
- Proc Natl Acad Sci U S A. 2008 Dec 9;105(49):19561. Rutschmann, Sophie [added]
Abstract
A recessive phenotype called spin (spontaneous inflammation) was induced by N-ethyl-N-nitrosourea (ENU) mutagenesis in C57BL/6J mice. Homozygotes display chronic inflammatory lesions affecting the feet, salivary glands and lungs, and antichromatin antibodies. They are immunocompetent and show enhanced resistance to infection by Listeria monocytogenes. TLR-induced TNF and IL-1 production are normal in macrophages derived from spin mice. The autoinflammatory phenotype of spin mice is fully suppressed by compound homozygosity for Myd88(poc), Irak4(otiose), and Il1r1-null mutations, but not Ticam1(Lps2), Stat1(m1Btlr), or Tnf-null mutations. Both autoimmune and autoinflammatory phenotypes are suppressed when spin homozygotes are derived into a germ-free environment. The spin phenotype was ascribed to a viable hypomorphic allele of Ptpn6, which encodes the tyrosine phosphatase SHP1, mutated in mice with the classical motheaten alleles me and me-v. Inflammation and autoimmunity caused by SHP1 deficiency are thus conditional. The SHP1-deficient phenotype is driven by microbes, which activate TLR signaling pathways to elicit IL-1 production. IL-1 signaling via MyD88 elicits inflammatory disease.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
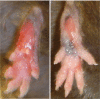
Fig. 1.
Chronic inflammation in homozygous spin mutants. Foot lesions develop in spin homozygotes from 6 weeks of age. Two representative foot lesions from spin homozygotes are shown.
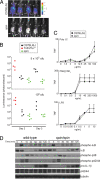
Fig. 2.
spin mice display increased resistance to L. monocytogenes. (A) Bacterial load was visualized in spin homozygotes and C57BL/6J mice 3 days after challenge with 5 × 105 cfu luminescent L. monocytogenes. Visual inspection and quantitation of luminescence demonstrated that spin homozygotes had reduced levels of bacteria compared to controls. (B) C57BL/6J, homozygous spin, or _Tnfrsf1a_-deficient mice were challenged with 5 × 105 or 106 cfu L. monocytogenes. The bacterial load, depicted as luminescence in photons per second, was determined 2 and 3 days after infection. _Tnfrsf1a_-deficient mice succumbed within 3 days of infection. One-way ANOVA and post-hoc Student-Newman-Keuls test, P < 0.05 for C57BL/6J vs. spin homozygotes on day 3 after infection with 5 × 105 cfu, and P < 0.05 for spin homozygous or C57BL/6J vs. _Tnfrsf1a_−/− mice on day 2 after infection with 106 cfu. (C) TNF production by peritoneal macrophages isolated from C57BL/6J or spin homozygous mice was measured in vitro in response to treatment with the indicated concentrations of TLR3, TLR2/1, or TLR4 ligands (poly I:C, Pam3CSK4, or lipopolysaccharide, respectively). TNF production was comparable between C57BL6/J and spin macrophages. The average response of cells from seven C57BL6/J and seven spin homozygous mice is plotted; error bars represent SD. (D) LPS-stimulated pro-IL-1β production, IκB degradation, and phosphorylation of MAP kinases p38, p42, and p44 in bone marrow-derived macrophages from C57BL/6J or spin homozygous mice were compared by Western blotting of lysates made at the indicated timepoints after LPS treatment in vitro. WT and spin macrophages responded similarly to LPS stimulation.
Fig. 3.
Detection of the spin mutation. (A) The mutation was predicted to introduce a HincII restriction enzyme site in genomic DNA from spin mutant mice. Amplification of Ptpn6 genomic DNA followed by HincII restriction enzyme digest confirmed the presence of the mutation. (B) SHP1 protein levels were normal in purified bone marrow-derived macrophages from spin homozygotes. The Western blot shown in Fig. 2_D_ was reprobed with SHP1 antibodies, or tubulin antibodies as a loading control. Timepoints indicate minutes after stimulation with LPS in vitro.
Fig. 4.
Autoimmune disease depends on MyD88 in spin mice. (A–D) The levels of serum polyclonal IgM (A) or IgG (B), and antichromatin IgM (C) or antichromatin IgG (D) were measured in 4- to 5-month-old mice of various genotypes. Compound homozygosity for Ptpn6 spin and Myd88 poc suppressed the elevated levels of serum immunoglobulins and antichromatin immunoglobulins found in Ptpn6 spin/spin mice. **, one-way ANOVA; P < 0.0001 and posthoc Student–Newman–Keuls test, P < 0.05 for Ptpn6spin/spinMyd88 poc/+ vs. all other genotypes.
Fig. 5.
Incidence curve showing age of onset of plantar inflammation in homozygous spin mice with or without null mutations in Il1r1. For difference between Il1r1+/+ and _Il1r1_−/−, P < 0.0001.
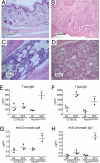
Fig. 6.
Chronic inflammation and autoimmune disease in homozygous spin mice depends on microbes. (A–D) Haematoxylin/eosin-stained tissue sections from 6- to 20-week-old Ptpn6 spin/spin mice derived and housed in GF conditions (A and C) and then conventionalized into normal SPF conditions (B and D). The feet of conventionalized mice are inflamed, with epidermal ulcerations displaying superficial necrosis and a leukocyte infiltrate (B), whereas GF mice display no foot inflammation (A). The cellularity of the bone marrow is increased in conventionalized mice, with numerous hematopoietic cells and granuloma-like structures (D). The bone marrow of GF mice appears normal (C). Sections are magnified ×20 (A and B) and ×40 (C and D). (E–H) The levels of serum polyclonal IgM (E) or IgG (F) and antichromatin IgM (G) or antichromatin IgG (H) were measured 6–12 weeks after mice housed in GF conditions were conventionalized by introduction into SPF conditions. GF conditions suppressed the elevated levels of immunoglobulins and anti-chromatin immunoglobulins found in Ptpn6 spin/spin mice housed in SPF conditions. **, Student's t test, P < 0.05 for GF Ptpn6 spin/spin mice vs. specific pathogen-free Ptpn6 spin/spin mice.
Similar articles
- Tyrosine Kinase SYK Licenses MyD88 Adaptor Protein to Instigate IL-1α-Mediated Inflammatory Disease.
Gurung P, Fan G, Lukens JR, Vogel P, Tonks NK, Kanneganti TD. Gurung P, et al. Immunity. 2017 Apr 18;46(4):635-648. doi: 10.1016/j.immuni.2017.03.014. Epub 2017 Apr 11. Immunity. 2017. PMID: 28410990 Free PMC article. - Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice.
Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Abram CL, et al. Immunity. 2013 Mar 21;38(3):489-501. doi: 10.1016/j.immuni.2013.02.018. Immunity. 2013. PMID: 23521885 Free PMC article. - B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity.
Pao LI, Lam KP, Henderson JM, Kutok JL, Alimzhanov M, Nitschke L, Thomas ML, Neel BG, Rajewsky K. Pao LI, et al. Immunity. 2007 Jul;27(1):35-48. doi: 10.1016/j.immuni.2007.04.016. Epub 2007 Jun 28. Immunity. 2007. PMID: 17600736 - Shp1 function in myeloid cells.
Abram CL, Lowell CA. Abram CL, et al. J Leukoc Biol. 2017 Sep;102(3):657-675. doi: 10.1189/jlb.2MR0317-105R. Epub 2017 Jun 12. J Leukoc Biol. 2017. PMID: 28606940 Free PMC article. Review. - Experimental and natural infections in MyD88- and IRAK-4-deficient mice and humans.
von Bernuth H, Picard C, Puel A, Casanova JL. von Bernuth H, et al. Eur J Immunol. 2012 Dec;42(12):3126-35. doi: 10.1002/eji.201242683. Eur J Immunol. 2012. PMID: 23255009 Free PMC article. Review.
Cited by
- Toll-like receptor signaling pathway involved in pathogenesis of thromboangiitis obliterans through activating of NF-κB.
Guo F, Bi Y, Yin J, Guo Y. Guo F, et al. Clinics (Sao Paulo). 2024 Apr 18;79:100357. doi: 10.1016/j.clinsp.2024.100357. eCollection 2024. Clinics (Sao Paulo). 2024. PMID: 38640750 Free PMC article. - Platelet ITGA2B inhibits caspase-8 and Rip3/Mlkl-dependent platelet death though PTPN6 during sepsis.
Jiang J, Li W, Zhou L, Liu D, Wang Y, An J, Qiao S, Xie Z. Jiang J, et al. iScience. 2023 Jul 19;26(8):107414. doi: 10.1016/j.isci.2023.107414. eCollection 2023 Aug 18. iScience. 2023. PMID: 37554440 Free PMC article. - Loss of Shp1 impairs myeloid cell function and causes lethal inflammation in zebrafish larvae.
Allers M, Bakker PA, Hoeksma J, Spaink HP, den Hertog J. Allers M, et al. Dis Model Mech. 2023 Feb 1;16(2):dmm049715. doi: 10.1242/dmm.049715. Epub 2023 Jan 30. Dis Model Mech. 2023. PMID: 36645087 Free PMC article. - SHP-1 tyrosine phosphatase binding to c-Src kinase phosphor-dependent conformations: A comparative structural framework.
Gul M, Navid A, Fakhar M, Rashid S. Gul M, et al. PLoS One. 2023 Jan 13;18(1):e0278448. doi: 10.1371/journal.pone.0278448. eCollection 2023. PLoS One. 2023. PMID: 36638102 Free PMC article.
References
- Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. - PubMed
- Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. - PubMed
- Shinkai K, McCalmont TH, Leslie KS. Cryopyrin-associated periodic syndromes and autoinflammation. Clin Exp Dermatol. 2008;33:1–9. - PubMed
- Shultz LD, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM067759/GM/NIGMS NIH HHS/United States
- HHSN272200700038C/PHS HHS/United States
- R01 GM067759/GM/NIGMS NIH HHS/United States
- R37 GM067759/GM/NIGMS NIH HHS/United States
- HHSN272200700038C/AI/NIAID NIH HHS/United States
- CA31496/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous