Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission - PubMed (original) (raw)
Superfluous role of mammalian septins 3 and 5 in neuronal development and synaptic transmission
Christopher W Tsang et al. Mol Cell Biol. 2008 Dec.
Abstract
The septin family of GTPases, first identified for their roles in cell division, are also expressed in postmitotic tissues. SEPT3 (G-septin) and SEPT5 (CDCrel-1) are highly expressed in neurons, enriched in presynaptic terminals, and associated with synaptic vesicles. These characteristics suggest that SEPT3 or SEPT5 might be important for synapse formation, maturation, or synaptic vesicle traffic. Since Sept5(-/-) mice do not show any overt neurological phenotypes, we generated Sept3(-/-) and Sept3(-/-) Sept5(-/-) mice and found that SEPT3 and SEPT5 are not essential for development, fertility, or viability. Changes in the expression of septins were noted in the absence of SEPT3, SEPT5, and both septins. SEPT5 association with other septins in brain tissue was unaffected by the removal of SEPT3. No abnormalities were observed in the gross morphology and synapses of the hippocampus. Similarly, axon development and synapse formation were unaffected in vitro. In cultured hippocampal neurons, the size of the recycling synaptic vesicle pool was unaltered in the absence of SEPT3. Furthermore, synaptic transmission at two different central synapses was not significantly affected in Sept3(-/-) Sept5(-/-) mice. These results indicate that SEPT3 and SEPT5 are dispensable for neuronal development as well as for synaptic vesicle fusion and recycling.
Figures
FIG. 1.
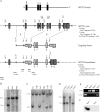
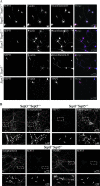
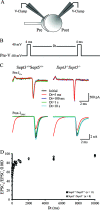
Gene targeting strategy, ES screening, and genotyping. (A) The mouse Sept3 gene was disrupted by replacing exon 2 to exon 5 with a Neor selection cassette. The cassette was targeted to the Sept3 locus by appending DNA fragments that were homologous to the Sept3 gene (targeting vector). A large piece of intron 1 and a small piece of exon 2 were used for the short arm of homology, whereas a piece of intron 5 through to the end of intron 8 was used for the large arm of homology. The endogenous and recombinant loci are shown above and below the targeting vector, respectively. For perspective, the location of relevant protein domains in relation to the encoding exons is shown at the top (PRO is the proline-rich domain, G1 is the P-loop motif, and G3 and G4 represent the other GTP binding features). Screening for recombination events was performed by Southern blotting using the 5′ and 3′ probes or by PCR using the Ex5-Ex6 and Ex2-Neo primer pairs. The sizes of DNA fragments that each probe is expected to detect by Southern blotting or that each primer pair is expected to amplify during PCR are shown on the right. The locations of only the relevant restriction enzyme cut sites are indicated for simplicity. (B) Screening results for proper homologous recombination of the short arm of homology in ES cells. Among the three ES cell clones shown, only clone 9F1 was targeted correctly. (C) Screening results for proper homologous recombination of the long arm of homology in ES cells. Out of six ES cell clones shown, only clones 9F1, 2A2, 11E2, and 12E2 were targeted correctly. (D) Genotype determined by Southern blot analysis of mouse tails from an F2 generation. (E) Genotype determined by PCR analysis of mouse tails from an F2 generation. (F) Western blot analysis of 20 μg of brain tissue from wild-type and _Sept3_−/− mice using an antibody to SEPT3.
FIG. 2.
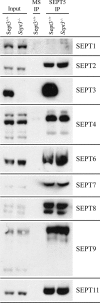
Lack of SEPT3 does not alter the association of other septins with SEPT5. Detergent-solubilized brain lysates from wild-type and _Sept3_−/− mice are shown in the first and second lanes, respectively. After the SEPT5 monoclonal antibody (SP20) was used to immunoprecipitate SEPT5 from wild-type or from _Sept3_−/− brain lysates, the coimmunoprecipitating septins were detected by immunoblotting (lanes 4 and 5). Mouse serum (MS) was used as a negative control for the immunoprecipitation (IP) procedure (lane 3). The input brain lysate together with the immunoprecipitates were separated by SDS-polyacrylamide gel electrophoresis, transferred onto PVDF membrane, and probed with the indicated septin antibodies.
FIG. 3.
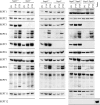
Levels of septin expression in the absence of SEPT3, SEPT5, and both septins. Brains from wild-type, _Sept3_−/−, _Sept5_−/−, and _Sept3_−/− _Sept5_−/− mice at P0, P14, and P28 were homogenized and then solubilized in SDS gel sample buffer. In each lane, 20 μg of protein sample was separated by SDS-polyacrylamide gel electrophoresis, transferred onto PVDF membrane, and probed with the indicated septin antibodies. A number of the septins undergo alterative splicing to produce multiple isoforms of different sizes on gels.
FIG. 4.
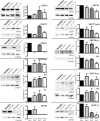
Quantitation of septin expression in the absence of SEPT3, SEPT5, and both septins. P30 brains from wild-type, _Sept3_−/−, _Sept5_−/−, and _Sept3_−/− _Sept5_−/− mice were homogenized and solubilized in SDS gel sample buffer. In each lane, 30 μg of protein sample was separated by SDS-polyacrylamide gel electrophoresis, transferred onto a PVDF membrane, and probed with the indicated septin antibody followed by the GAPDH antibody. The relative level of each septin after calibration was measured in three independent experiments and averaged. Any significant deviation in septin expression from wild-type levels is indicated by an asterisk.
FIG. 5.
Loss of SEPT3 or SEPT3 and SEPT5 does not affect the development of primary hippocampal neurons in vitro. Primary mouse hippocampal neurons from wild-type, _Sept3_−/−, and _Sept3_−/− _Sept5_−/− brains were cultured for 3 days (A) or 10 days (B). Wild-type and _Sept3_−/− neurons cultured for 3 days were stained for SEPT3, F-actin, and neurofilament-H (green, red, and blue, respectively, in the merged panel). In neurons lacking SEPT3, processes containing the axon-specific protein neurofilament-H still form growth cones at their tips (white arrow). _Sept3_−/− _Sept5_−/− neurons cultured for 3 days were stained in the top series of panels for SEPT3, F-actin, and neurofilament-H (green, red, and blue, respectively, in the merged panel) or in the bottom series of panels for SEPT5, F-actin, and MAP2 (green, red, and blue, respectively, in the merged panel). Like _Sept3_−/− neurons, F-actin-rich growth cones at the tips of axons (arrow) are still present in the absence of SEPT3 and SEPT5. Wild-type and _Sept3_−/− neurons cultured for 10 days were stained for SEPT3 and synaptophysin. _Sept3_−/− _Sept5_−/− neurons cultured for 10 days were stained in the left series of panels for SEPT3 and synaptophysin or in the right series of panels for SEPT5 and VAMP2. A digitally magnified image of the region highlighted by the hatched box is shown directly below. The arrows point to presynaptic terminals enriched with synaptophysin. In neurons lacking SEPT3 or SEPT3 and SEPT5, many punctate regions enriched with synaptophysin or VAMP2 still form (white arrows).
FIG. 6.
Hippocampal anatomy in wild-type, _Sept3_−/−, _Sept5_−/−, and _Sept3_−/− _Sept5_−/− brains. (A) A series of magnified images of the hippocampus from wild-type, _Sept3_−/−, _Sept5_−/−, and _Sept3_−/− _Sept5_−/− mice. Pictures were taken from sagittal sections of formalin-fixed, P30 brains that were stained with hematoxylin and eosin. The bubbles and large spaces around each hippocampus were a result of artifacts that were introduced during tissue preparation. (B) A series of electron transmission micrographs that were taken of nerve terminals from the CA1 region of the hippocampus (boxed area illustrated in panel A). Two representative examples from each genotype are shown.
FIG. 7.
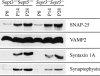
Expression of synaptic vesicle proteins in the absence of SEPT3 and SEPT5. Brains from wild-type and _Sept3_−/− _Sept5_−/− mice at P0, P14, and P28 were homogenized and then solubilized in SDS gel sample buffer. In each lane, 20 μg of protein sample was separated by SDS-polyacrylamide gel electrophoresis, transferred onto PVDF membrane, and probed with the indicated antibodies.
FIG. 8.
Loss of SEPT3 does not affect paired-pulse facilitation or the amplitude and frequency of spontaneous EPSCs in hippocampal CA1 pyramidal neurons. (A) The top trace represents superimposed AMPA receptor-mediated EPSCs from a wild-type mouse evoked by paired stimuli (150 ms apart). The bottom trace represents an average of 15 consecutive traces. (B) The top trace represents superimposed AMPA receptor-mediated EPSCs from a _Sept3_−/− _Sept5_−/− mouse evoked by paired stimuli (150 ms apart). The bottom trace represents an average of 15 consecutive traces. (C) A representative 4-s segment of spontaneous EPSCs recorded from a wild-type mouse. A blow-up of the postsynaptic currents is shown in the inset (inset scale, 10 pA/6 ms). (D) A representative 4-s segment of spontaneous EPSCs recorded from a _Sept3_−/− _Sept5_−/− mouse. A blow-up of the postsynaptic currents is shown in the inset (inset scale, 10 pA/6 ms). (E) Pooled data showing the PPR of EPSCs for wild-type and _Sept3_−/− _Sept5_−/− mice. (F) Pooled data showing frequency of spontaneous EPSCs for wild-type and _Sept3_−/− _Sept5_−/− mice. (G) Pooled data showing average amplitude for spontaneous EPSCs for wild-type and _Sept3_−/− _Sept5_−/− mice. Error bars in panels E, F, and G show SEMs.
FIG. 9.
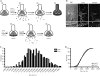
Synaptic vesicle recycling pool size in _Sept3_−/− neurons. (A) FM 1-43 loading and unloading protocol used to measure the size of the synaptic vesicle recycling pool. The diagram outlines the steps involved in loading and unloading presynaptic terminals of FM 1-43. (B) Differential interference contrast (DIC) image of a wild-type mouse primary hippocampal neuron cultured for 10 days from a wild-type mouse (left panel). A fluorescence image of the same wild-type neuron studded with FM 1-43-loaded nerve terminals is shown in the middle panel. A fluorescence image of the same wild-type neuron after unloading nerve terminals of FM 1-43 by K+ depolarization is shown in the right panel. A magnified view of the region highlighted by the hatched box is shown directly below. (C) The size of the recycling pool was measured individually from each synapse, and the data were plotted as a frequency distribution. The distributions of the recycling pool size from wild-type and _Sept3_−/− nerve terminals is represented by the open bars and the closed bars, respectively (n = 532 terminals from seven wild-type pups and n = 555 terminals from six _Sept3_−/− pups). (D) A cumulative frequency curve was constructed for statistical analysis from the distribution of recycling pool sizes in panel C. Data from wild-type nerve terminals are plotted with squares, whereas data from _Sept3_−/− nerve terminals are plotted with triangles. A.F.U., arbitrary fluorescence units.
FIG. 10.
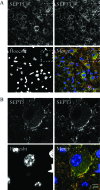
SEPT3 and SEPT5 are expressed in the calyx of Held nerve terminal. (A) A brain slice from the auditory cortex of a P16 mouse was stained for SEPT3 (green), SEPT5 (red), and DNA (Hoechst; blue). A digitally magnified image of the region highlighted by the hatched box is shown in panel B below.
FIG. 11.
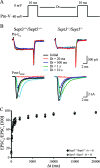
Loss of SEPT3 and SEPT5 does not affect the replenishment of the immediately available pool of synaptic vesicles. (A) Schematic illustration of the paired voltage clamp recording arrangement from the calyx of Held-MNTB synapse. (B) Presynaptic voltage clamp command paradigm used to measure recovery of the portion of the RRP of synaptic vesicles available in response to a single action potential. Two voltage steps (−80 to 40 mV; 4 ms), separated by increasing Δ_t_ ([Dt] intervals ranging from 5 ms to 10 s), were used to evoke _I_Ca and _I_EPSC from voltage-clamped presynaptic calyces and their corresponding postsynaptic MNTB neurons. (C) Presynaptic _I_Ca (top) and postsynaptic _I_EPSC (bottom) recorded from wild-type (left) and _Sept3_−/− _Sept5_−/− (right) synapses and superimposed at selected interstep intervals (Dt) in response to the presynaptic voltage command shown in panel A. (D) Pooled data for wild-type (closed circles) and _Sept3_−/− _Sept5_−/− (open diamonds) plotting recovery of the _I_EPSC area against the interstep interval (Dt). Error bars show SEMs.
FIG. 12.
Loss of SEPT3 and SEPT5 does not affect replenishment of the readily releasable pool of synaptic vesicles. (A) Presynaptic voltage-clamp command paradigm used to measure recovery of the readily releasable pool of synaptic vesicles. Two voltage steps (−80 to 0 mV; 10 ms), separated by increasing Δ_t_ ([Dt] intervals ranging from 20 ms to 20 s), were used to evoke _I_Ca and _I_EPSC from voltage-clamped presynaptic calyces and their corresponding postsynaptic MNTB neurons. (B) Presynaptic _I_Ca (top) and postsynaptic _I_EPSC (bottom), recorded from wild-type (left) and _Sept3_−/− _Sept5_−/− (right) synapses and superimposed, at selected interstep intervals (Dt) in response to the presynaptic voltage command shown in panel B. (C) Pooled data for wild-type (closed circles) and _Sept3_−/− _Sept5_−/− (open diamonds) plotting recovery of I_EPSC area against interstep interval (Δ_t). Error bars show SEMs.
Similar articles
- Targeted disruption of Sept3, a heteromeric assembly partner of Sept5 and Sept7 in axons, has no effect on developing CNS neurons.
Fujishima K, Kiyonari H, Kurisu J, Hirano T, Kengaku M. Fujishima K, et al. J Neurochem. 2007 Jul;102(1):77-92. doi: 10.1111/j.1471-4159.2007.04478.x. J Neurochem. 2007. PMID: 17564677 - Septin 3 (G-septin) is a developmentally regulated phosphoprotein enriched in presynaptic nerve terminals.
Xue J, Tsang CW, Gai WP, Malladi CS, Trimble WS, Rostas JA, Robinson PJ. Xue J, et al. J Neurochem. 2004 Nov;91(3):579-90. doi: 10.1111/j.1471-4159.2004.02755.x. J Neurochem. 2004. PMID: 15485489 - Characterization of presynaptic septin complexes in mammalian hippocampal neurons.
Tsang CW, Estey MP, DiCiccio JE, Xie H, Patterson D, Trimble WS. Tsang CW, et al. Biol Chem. 2011 Aug;392(8-9):739-49. doi: 10.1515/BC.2011.077. Epub 2011 Jul 19. Biol Chem. 2011. PMID: 21767234 - Mammalian septin function in hemostasis and beyond.
Martinez C, Ware J. Martinez C, et al. Exp Biol Med (Maywood). 2004 Dec;229(11):1111-9. doi: 10.1177/153537020422901105. Exp Biol Med (Maywood). 2004. PMID: 15564437 Review. - Septins, a novel group of GTP-binding proteins: relevance in hemostasis, neuropathology and oncogenesis.
Roeseler S, Sandrock K, Bartsch I, Zieger B. Roeseler S, et al. Klin Padiatr. 2009 May-Jun;221(3):150-5. doi: 10.1055/s-0029-1220706. Epub 2009 May 12. Klin Padiatr. 2009. PMID: 19437362 Review.
Cited by
- SEPT3 as a Potential Molecular Target of Triple-Negative Breast Cancer.
Yang LH, Wang GZ, Gao C. Yang LH, et al. Int J Gen Med. 2024 Apr 25;17:1605-1613. doi: 10.2147/IJGM.S462541. eCollection 2024. Int J Gen Med. 2024. PMID: 38686040 Free PMC article. - SEPTIN12 genetic variants confer susceptibility to teratozoospermia.
Lin YH, Wang YY, Chen HI, Kuo YC, Chiou YW, Lin HH, Wu CM, Hsu CC, Chiang HS, Kuo PL. Lin YH, et al. PLoS One. 2012;7(3):e34011. doi: 10.1371/journal.pone.0034011. Epub 2012 Mar 30. PLoS One. 2012. PMID: 22479503 Free PMC article. - Septins regulate developmental switching from microdomain to nanodomain coupling of Ca(2+) influx to neurotransmitter release at a central synapse.
Yang YM, Fedchyshyn MJ, Grande G, Aitoubah J, Tsang CW, Xie H, Ackerley CA, Trimble WS, Wang LY. Yang YM, et al. Neuron. 2010 Jul 15;67(1):100-15. doi: 10.1016/j.neuron.2010.06.003. Neuron. 2010. PMID: 20624595 Free PMC article. - Septin dynamics are essential for exocytosis.
Tokhtaeva E, Capri J, Marcus EA, Whitelegge JP, Khuzakhmetova V, Bukharaeva E, Deiss-Yehiely N, Dada LA, Sachs G, Fernandez-Salas E, Vagin O. Tokhtaeva E, et al. J Biol Chem. 2015 Feb 27;290(9):5280-97. doi: 10.1074/jbc.M114.616201. Epub 2015 Jan 9. J Biol Chem. 2015. PMID: 25575596 Free PMC article. - Neuronal-specific septin-3 binds Atg8/LC3B, accumulates and localizes to autophagosomes during induced autophagy.
Tóth V, Vadászi H, Ravasz L, Mittli D, Mátyás D, Molnár T, Micsonai A, Szaniszló T, Lőrincz P, Kovács RÁ, Juhász T, Beke-Somfai T, Juhász G, Györffy BA, Kékesi KA, Kardos J. Tóth V, et al. Cell Mol Life Sci. 2022 Aug 6;79(9):471. doi: 10.1007/s00018-022-04488-8. Cell Mol Life Sci. 2022. PMID: 35932293 Free PMC article.
References
- Ahuja, P., E. Perriard, W. Trimble, J. C. Perriard, and E. Ehler. 2006. Probing the role of septins in cardiomyocytes. Exp. Cell Res. 3121598-1609. - PubMed
- Barr, A. M., C. E. Young, K. Sawada, W. S. Trimble, A. G. Phillips, and W. G. Honer. 2004. Abnormalities of presynaptic protein CDCrel-1 in striatum of rats reared in social isolation: relevance to neural connectivity in schizophrenia. Eur. J. Neurosci. 20303-307. - PubMed
- Beites, C. L., H. Xie, R. Bowser, and W. S. Trimble. 1999. The septin CDCrel-1 binds syntaxin and inhibits exocytosis. Nat. Neurosci. 2434-439. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases