Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster - PubMed (original) (raw)
Ero1L, a thiol oxidase, is required for Notch signaling through cysteine bridge formation of the Lin12-Notch repeats in Drosophila melanogaster
An-Chi Tien et al. J Cell Biol. 2008.
Abstract
Notch-mediated cell-cell communication regulates numerous developmental processes and cell fate decisions. Through a mosaic genetic screen in Drosophila melanogaster, we identified a role in Notch signaling for a conserved thiol oxidase, endoplasmic reticulum (ER) oxidoreductin 1-like (Ero1L). Although Ero1L is reported to play a widespread role in protein folding in yeast, in flies Ero1L mutant clones show specific defects in lateral inhibition and inductive signaling, two characteristic processes regulated by Notch signaling. Ero1L mutant cells accumulate high levels of Notch protein in the ER and induce the unfolded protein response, suggesting that Notch is misfolded and fails to be exported from the ER. Biochemical assays demonstrate that Ero1L is required for formation of disulfide bonds of three Lin12-Notch repeats (LNRs) present in the extracellular domain of Notch. These LNRs are unique to the Notch family of proteins. Therefore, we have uncovered an unexpected requirement for Ero1L in the maturation of the Notch receptor.
Figures
Figure 1.
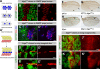
Alleles of kiga cause bristle tufting and wing defects. (A) The bristle and socket cells are external structures of a mechanosensory organ (arrowhead). They are present on the head epidermis of a wild-type (WT) fly at stereotypic positions. (B) Homozygous kiga mutant clones show a bristle-tufting phenotype indicating a defect in lateral inhibition. Compared with the regularly spaced bristle patterning on the wild-type head epidermis (A), clear expansion of bristles are observed in kiga clones induced by ey-FLP on the head epidermis (arrowheads). (C) Bristle patterning on the thorax is also affected in homozygous kiga mutant clones. Homozygous mutant kiga clones induced by Ubx-FLP are identified by the trichome marker multiple wing hairs and marked by dashed lines. A higher magnification of a mutant clone in C is shown in C'. (D and E) Wing formation is affected in homozygous kiga mutant clones. Compared with wild-type wing (D), loss of wing tissue around the wing margin (arrow) and wing vein-thickening phenotypes (arrowhead) are associated with homozygous kiga mutant clones (E).
Figure 2.
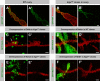
kiga is specifically required for Notch-dependent processes and interacts genetically with Notch mutants. (A) Schematic illustration of the lateral inhibition process mediated by Notch signaling. (A') Schematic illustration of inductive signaling. The “N's” in the cells represent high Notch activity. The wing margin cell fate (blue) is induced by Notch signaling between dorsal and ventral compartments (yellow and orange). In B–E and J–K, homozygous mutant regions lack GFP expression (green). (B) Lateral inhibition is impaired in homozygous kiga mutant clones. The SOPs of the pupal notum at 12 h APF are labeled for Sens (red). (B and B') Note that SOPs in the wild-type region are spaced regularly between epithelial cells, whereas no epithelial cells are present between mutant SOPs. (C and C') Binary fate decision at 24 h APF in kiga mutant clones. Cut (blue) marks all of the cells of the SOP progeny. Elav (red) stains the neuronal cells. In the wild-type domain, there are well-spaced clusters of four Cut-positive cells and one Elav-positive cell. In kiga mutant clones, Cut expression is expanded, and it is difficult to identify single clusters, indicating a lateral inhibition defect. However, the presence of neuronal cells suggests that cell fate specification and differentiation are likely normal. (D and D') Defective wing margin formation is associated with kiga mutants. Wing imaginal disc from a third instar larva stained for Cut (red). Cells in the large homozygous mutant clones lack expression of Cut at the DV boundary. A higher magnification of a small portion of D is shown in E and E'. Note that in large clones, mutant cells at the boundary of the clone are not Cut positive (arrow), whereas in some smaller mutant clones and near the edge of wild-type cells, some margin cells are Cut positive (*). (F and G) Removal of one copy of kiga suppresses a gain of function phenotype of Notch. A male adult wing from an NAxE-2/Y fly is shown (F) with a reduced length of veins (F, arrows). The loss of wing veins is suppressed (G, arrows) when one copy of kiga is removed in NAx E-2/Y; kiga/+ male wings (G). (H and I) Removal of one copy of kiga does not suppress a gain of function phenotype of Egfr. A male adult wing from an Elp/+ fly is shown (H) with an ectopic wing vein (H, arrow). The gain of wing vein (I, arrow) is not suppressed when one copy of kiga is removed. (J–K') Dpp and Hh signaling are unaffected in kiga mutant clones. Wing imaginal disc with kiga mutant clones stained for phospho-Mad (J and J', red) and Ci (K and K', red). Bars: (B–C') 10 μm; (D–E' and J–K') 40 μm.
Figure 3.
kiga is required in signal-receiving cells for Notch signaling. (A–B') Wild-type (WT) ovary (A and A') and ovary with kiga mutant clones (B and B') were stained with phalloidin (red) to outline the cell boundary. (B and B') A large kiga mutant clone marked by loss of GFP expression (green) results in a fused egg chamber phenotype. (C–F') Notch signaling activity reported by Cut expression at the DV boundary using the MARCM technique. Part of the wing discs stained for Cut (red) is shown, and the mutant region is marked positively with GFP expression (green). (C and C') Overexpression of Dl in kiga mutant cells can induce Cut expression in the adjacent cells near the DV boundary at the dorsal compartment. (D and D') Overexpression of Notch in wild-type cells can induce Cut expression cell autonomously near the DV boundary. (E and E') Overexpression of Notch in kiga mutant cells fails to induce Cut expression near the DV boundary. Note that when the clones are crossing the boundary, Cut expression is lost in the mutant cells. (F and F') Overexpression of NEXT in kiga mutant cells can induce Cut expression cell autonomously. Bars: (A–B') 20 μm; (C–F') 10 μm.
Figure 4.
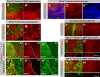
Mutations in Ero1L cause the kiga mutant phenotypes. (A) Schematic illustration of Ero1L and neighboring genes in chromosomal region 64A9. (B) Position of the stop codons (indicated by asterisks) in four Ero1L alleles; 23T and 335QRS were generated in the screen in this study, whereas others were isolated previously in a screen for essential complementation groups in the 63E-64A region (Harrison et al., 1995). Complementation tests and sequencing revealed that they are alleles of kiga. (C) Overexpression of Ero1L with tub-GAL4 rescues the tufting phenotype associated with kiga mutant clones. A thorax of an adult fly harboring kiga mutant clones (marked by dashed lines) expressing the Ero1L cDNA is shown. (D) The rescued region is shown at higher magnification. (E and F) Only the enzymatically active form of the human homologue of Ero1L can rescue the tufting phenotype in Ero1L mutant clones. A thorax of an adult fly harboring Ero1L clones (marked by dashed line) expressing a wild-type human ERO1-Lα (E) or C394A mutant (F) cDNA is shown. Only the enzymatically active (wild type) form of human ERO1-Lα restores normal bristle patterning (E). Conversely, Ero1L mutant clones expressing enzymatically inactive human ERO1Lα (C394A) exhibit tufting phenotypes (F).
Figure 5.
Ero1L mutant cells exhibit a UPR and accumulate Notch in the ER. (A–H) Immunostainings of the pupal nota (A and A' and G–H') or wing imaginal discs (B–F') that harbor _Ubx-FLP_-induced Ero1L homozygous clones (A–E” and G–H', marked by lack of GFP expression). (A) Ero1L mutant cells exhibit a UPR. Pupal notum (12 h APF) labeled with Hsc3 (red). Significantly higher levels of Hsc3 expression are observed in mutant tissue. (B and B') Cell-autonomous up-regulation of Notch in Ero1L mutant clones. Wing imaginal disc stained with an antibody raised against the intracellular portion of Notch (red). Much higher levels of Notch are observed in Ero1L mutant cells when compared with wild-type cells. Note that accumulation of Notch near the DV boundary (arrows) is less pronounced. (C–D”) Accumulation of Notch colocalizes with ER markers in Ero1L mutant cells. Wing imaginal disc stained for Notch (red) and Boca (green), an ER chaperone. Apical honeycomb-like localization of Notch is lost in the Ero1L mutant clones (C–C”), whereas the basal section shows that Notch is accumulated intracellularly and colocalized with Boca (D–D”). The dotted lines mark the clone boundary. (E–E”) Membrane-bound Notch is severely decreased in Ero1L mutant cells. Wing imaginal disc stained with an antibody raised against the NECD (E', red; no permeabilization). Anti-Notch staining performed after permeabilization shows a very strong intracellular accumulation of Notch (E”, blue). (F) Up-regulation of a mutant Hsc3 known to induce a UPR does not affect the levels or localization of Notch. Wing imaginal disc overexpressing mutant Hsc3 (D231S) in the AP boundary driven by patched-GAL4 were stained for Notch (red) and Hsc3 (green). No accumulation or mislocalization of Notch in the patched expression domain is observed. (G–H') Different levels of Notch accumulation are observed at different developmental stages in mutant cells. Wing imaginal disc at the third instar larval stage (B and B') or pupal nota at 12 h APF (G and G') and 24 h APF (H and H') were stained for Notch (red). The bottom panels of G–H' show the z-section slide. Note that significantly less accumulated Notch is present at 24 h APF (when four SOP progeny initiate differentiation) in mutant cells relative to wild-type cells when compared with 12 h APF, the time point when lateral inhibition is terminating. Bars, 10 μm.
Figure 6.
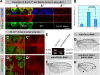
Notch is a major target in Ero1L mutant cells. (A–D') Confocal sections through wing imaginal discs harboring _Ubx-FLP_–induced mutant clones (lack of GFP expression). (A–A'”) Cells without Notch and Ero1L have lower levels of Hsc3 expression compared with cells that lack only Ero1L. A z section through a wing imaginal disc harboring Ero1L and/or Notch mutant clones (marked by dashed lines) is shown. It was stained for Notch (green) and Hsc3 (red). In A”, wild-type cells expressing GFP (blue) show low levels of expression for both Hsc3 and Notch. In A', high levels of Hsc3 and Notch are observed in cells that are only mutant for Ero1L. In A”', a group of Notch and Ero1L double mutant cells, indicated by the loss of GFP (blue) and Notch (green) expression, show lower levels of Hsc3 when compared with Ero1L mutant cells in the left panel (compare A'” with A'). (B) Quantification of Hsc3 expression in the cells of different genotypes is shown in the bar graph (a.u., arbitrary unit). Error bars indicate SEM. (C–D') Normal expression levels and localization of various membrane proteins are observed in Ero1L mutant clones. Dl (C–C', red) and Drosophila EGF receptor (D and D', red) are localized normally and are not up-regulated in Ero1L mutant cells. (E) Knockdown of CG4670 revealed by semiquantitative RT-PCR. In larva with ubiquitous expression of CG4670 RNAi (left), the mRNA of CG4670 is significantly lower than control larva (right). (F–H) Genetic interaction between QSOX protein and Ero1L. Wings from adult female flies incubated at 25°C. The genotype of each fly is indicated near the figure, and the arrows indicate the wing vein-thickening phenotypes. Bars, 10 μm.
Figure 7.
Ero1L is involved in secretion and disulfide bond formation of the LNR domain of Notch. (A) Secretion of the LNR domain into the medium is affected when Ero1L is reduced (lane 4, bottom, Western blot). S2 cells treated with either dsRNA against EGFP (lanes 1 and 3) or Ero1L (lanes 2 and 4) were followed by transfection with EGF-V5 (lanes 1 and 2) and LNR-V5 (lanes 3 and 4). Western blots of the cell lysate (top) and the medium from these samples are shown (bottom). (B) Secretion of the EGF domain into the medium is affected when QSOX1 (CG4670) is knocked down. S2 cells treated with various dsRNA are indicated in the table above the Western blotting. The bottom panel shows the relative abundance of mRNA of Arp66B (control), Ero1L, and QSOX1, which was measured by RT-PCR. (C) Disulfide bonds in the LNR domain of Notch fail to form properly in Ero1L knockdown S2 cells. An AMS thiol-modifying analysis was used to reveal disulfide bond formation under various conditions. As a positive control, lysate was treated with DTT followed by AMS incubation. The expected molecular weight shift of the LNR domain is observed in lane 6 (*). A similar molecular weight shift is observed in Ero1L knockdown lysate without prior DTT treatment (lane 4), indicating a loss of disulfide bond formation in a portion of the LNR domain. Note that the LNR domain can form homodimers without AMS treatment (**) that can be removed in a high concentration of urea (not depicted).
Similar articles
- A genetic mosaic screen identifies genes modulating Notch signaling in Drosophila.
Ren L, Mo D, Li Y, Liu T, Yin H, Jiang N, Zhang J. Ren L, et al. PLoS One. 2018 Sep 20;13(9):e0203781. doi: 10.1371/journal.pone.0203781. eCollection 2018. PLoS One. 2018. PMID: 30235233 Free PMC article. - Two pathways for importing GDP-fucose into the endoplasmic reticulum lumen function redundantly in the O-fucosylation of Notch in Drosophila.
Ishikawa HO, Ayukawa T, Nakayama M, Higashi S, Kamiyama S, Nishihara S, Aoki K, Ishida N, Sanai Y, Matsuno K. Ishikawa HO, et al. J Biol Chem. 2010 Feb 5;285(6):4122-4129. doi: 10.1074/jbc.M109.016964. Epub 2009 Nov 30. J Biol Chem. 2010. PMID: 19948734 Free PMC article. - Notch Missense Mutations in Drosophila Reveal Functions of Specific EGF-like Repeats in Notch Folding, Trafficking, and Signaling.
Nurmahdi H, Hasegawa M, Mujizah EY, Sasamura T, Inaki M, Yamamoto S, Yamakawa T, Matsuno K. Nurmahdi H, et al. Biomolecules. 2022 Nov 25;12(12):1752. doi: 10.3390/biom12121752. Biomolecules. 2022. PMID: 36551180 Free PMC article. - Regulation of notch signaling via O-glucosylation insights from Drosophila studies.
Lee TV, Takeuchi H, Jafar-Nejad H. Lee TV, et al. Methods Enzymol. 2010;480:375-98. doi: 10.1016/S0076-6879(10)80017-5. Methods Enzymol. 2010. PMID: 20816218 Review. - Epigenetic Regulation of Notch Signaling During Drosophila Development.
Wei C, Phang CW, Jiao R. Wei C, et al. Adv Exp Med Biol. 2020;1218:59-75. doi: 10.1007/978-3-030-34436-8_4. Adv Exp Med Biol. 2020. PMID: 32060871 Review.
Cited by
- dEHBP1 controls exocytosis and recycling of Delta during asymmetric divisions.
Giagtzoglou N, Yamamoto S, Zitserman D, Graves HK, Schulze KL, Wang H, Klein H, Roegiers F, Bellen HJ. Giagtzoglou N, et al. J Cell Biol. 2012 Jan 9;196(1):65-83. doi: 10.1083/jcb.201106088. Epub 2012 Jan 2. J Cell Biol. 2012. PMID: 22213802 Free PMC article. - A genetic mosaic screen identifies genes modulating Notch signaling in Drosophila.
Ren L, Mo D, Li Y, Liu T, Yin H, Jiang N, Zhang J. Ren L, et al. PLoS One. 2018 Sep 20;13(9):e0203781. doi: 10.1371/journal.pone.0203781. eCollection 2018. PLoS One. 2018. PMID: 30235233 Free PMC article. - Cooperative Protein Folding by Two Protein Thiol Disulfide Oxidoreductases and 1 in Soybean.
Matsusaki M, Okuda A, Masuda T, Koishihara K, Mita R, Iwasaki K, Hara K, Naruo Y, Hirose A, Tsuchi Y, Urade R. Matsusaki M, et al. Plant Physiol. 2016 Feb;170(2):774-89. doi: 10.1104/pp.15.01781. Epub 2015 Dec 8. Plant Physiol. 2016. PMID: 26645455 Free PMC article. - ERO1-beta, a pancreas-specific disulfide oxidase, promotes insulin biogenesis and glucose homeostasis.
Zito E, Chin KT, Blais J, Harding HP, Ron D. Zito E, et al. J Cell Biol. 2010 Mar 22;188(6):821-32. doi: 10.1083/jcb.200911086. J Cell Biol. 2010. PMID: 20308425 Free PMC article. - Oxidative protein folding by an endoplasmic reticulum-localized peroxiredoxin.
Zito E, Melo EP, Yang Y, Wahlander Å, Neubert TA, Ron D. Zito E, et al. Mol Cell. 2010 Dec 10;40(5):787-97. doi: 10.1016/j.molcel.2010.11.010. Mol Cell. 2010. PMID: 21145486 Free PMC article.
References
- Acar, M., H. Jafar-Nejad, N. Giagtzoglou, S. Yallampalli, G. David, Y. He, C. Delidakis, and H.J. Bellen. 2006. Senseless physically interacts with proneural proteins and functions as a transcriptional co-activator. Development. 133:1979–1989. - PubMed
- Artavanis-Tsakonas, S., M.D. Rand, and R.J. Lake. 1999. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. - PubMed
- Bardin, A.J., R. Le Borgne, and F. Schweisguth. 2004. Asymmetric localization and function of cell-fate determinants: a fly's view. Curr. Opin. Neurobiol. 14:6–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases