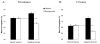Sleep preferentially enhances memory for emotional components of scenes - PubMed (original) (raw)
Sleep preferentially enhances memory for emotional components of scenes
Jessica D Payne et al. Psychol Sci. 2008 Aug.
Abstract
Central aspects of emotional experiences are often well remembered at the expense of background details. Previous studies of such memory trade-offs have focused on memory after brief delays, but little is known about how these components of emotional memories change over time. We investigated the evolution of memory for negative scenes across 30 min, 12 daytime hours spent awake, and 12 nighttime hours including sleep. After 30 min, negative objects were well remembered at the expense of information about their backgrounds. Time spent awake led to forgetting of the entire negative scene, with memories of objects and their backgrounds decaying at similar rates. Sleep, in contrast, led to a preservation of memories of negative objects, but not their backgrounds, a result suggesting that the two components undergo differential processing during sleep. Memory for a negative scene develops differentially across time delays containing sleep and wake, with sleep selectively consolidating those aspects of memory that are of greatest value to the organism.
Figures
Figure 1
Each set of 8 scenes was created from two versions of a neutral object (e.g., two cars), two versions of a negative and arousing emotional object (e.g., two car accidents), and two versions of a neutral background on which these objects could plausibly be found (e.g., two streets). Objects and backgrounds were used to create eight versions of a scene, representing all possible combinations of an object and a background (only two of which are shown here).
Figure 2
Mean overall recognition memory for objects (black bars), and backgrounds (white bars) for neutral (left) and negative emotional (right) scenes after 30 minutes and 12 hours (collapsed across wake and sleep groups). Negative emotional objects were well remembered at the expense of their (neutral) backgrounds (note the difference in height between the black and white bars for the negative scenes), which reflects the predicted central/peripheral trade-off in emotional memory for scenes. Arrows represent the average recall of objects and backgrounds in the neutral condition, and emphasize that negative objects are better recognized than neutral objects, while backgrounds containing emotional objects are more poorly recognized than backgrounds containing neutral objects. Overall recognition = same + similar responses to old stimuli – false alarms, after Kensinger et al. (2007). Note that because the Y-axes reflect overall recognition scores, they not directly comparable with Figure 3 (which splits memory into general and specific recognition).
Figure 3
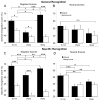
Mean recognition memory for objects (black bars), and backgrounds (white bars) for the three delay conditions – 30 minutes, wake, and sleep. (A) General recognition for negative scenes: memory for emotional objects is maintained and even slightly enhanced across sleep relative to 30 minutes (arrow), resulting in different patterns of negative scene memory components in the wake and sleep conditions; (B) General recognition for neutral scenes: both sleep and wake lead to roughly equivalent reductions in memory for objects and backgrounds, to values of 45±4%. Note that there are no significant differences between the sleep and wake groups, nor are there differences between objects and backgrounds at any of the three time delays; (C) Specific recognition for negative scenes: as with general recognition, negative objects are selectively maintained in the sleep condition; (D) Specific recognition for neutral scenes: as with general recognition, there are no significant differences between object and background recall at any delay, and none between the wake and sleep condition. But both wake and sleep delays show significantly poorer recall than at 30 min. Significant effects are denoted by asterisks (* p <.05, ** p ≤ .01, *** p ≤ .001).
Similar articles
- The differential effects of emotional salience on direct associative and relational memory during a nap.
Alger SE, Payne JD. Alger SE, et al. Cogn Affect Behav Neurosci. 2016 Dec;16(6):1150-1163. doi: 10.3758/s13415-016-0460-1. Cogn Affect Behav Neurosci. 2016. PMID: 27670288 - Psychophysiological arousal at encoding leads to reduced reactivity but enhanced emotional memory following sleep.
Cunningham TJ, Crowell CR, Alger SE, Kensinger EA, Villano MA, Mattingly SM, Payne JD. Cunningham TJ, et al. Neurobiol Learn Mem. 2014 Oct;114:155-64. doi: 10.1016/j.nlm.2014.06.002. Epub 2014 Jun 18. Neurobiol Learn Mem. 2014. PMID: 24952130 - The effects of cognitive reappraisal and sleep on emotional memory formation.
Martinez BS, Denis D, Kim SY, DiPietro CH, Stare C, Kensinger EA, Payne JD. Martinez BS, et al. Cogn Emot. 2023 Aug-Sep;37(5):942-958. doi: 10.1080/02699931.2023.2221843. Epub 2023 Jun 12. Cogn Emot. 2023. PMID: 37307073 - Sleep facilitates consolidation of emotional declarative memory.
Hu P, Stylos-Allan M, Walker MP. Hu P, et al. Psychol Sci. 2006 Oct;17(10):891-8. doi: 10.1111/j.1467-9280.2006.01799.x. Psychol Sci. 2006. PMID: 17100790 - The whats and whens of sleep-dependent memory consolidation.
Diekelmann S, Wilhelm I, Born J. Diekelmann S, et al. Sleep Med Rev. 2009 Oct;13(5):309-21. doi: 10.1016/j.smrv.2008.08.002. Epub 2009 Feb 28. Sleep Med Rev. 2009. PMID: 19251443 Review.
Cited by
- Tell me why: the missing w in episodic memory's what, where, and when.
Morales-Calva F, Leal SL. Morales-Calva F, et al. Cogn Affect Behav Neurosci. 2024 Oct 25. doi: 10.3758/s13415-024-01234-4. Online ahead of print. Cogn Affect Behav Neurosci. 2024. PMID: 39455523 - Elements of episodic memory: insights from artificial agents.
Boyle A, Blomkvist A. Boyle A, et al. Philos Trans R Soc Lond B Biol Sci. 2024 Nov 4;379(1913):20230416. doi: 10.1098/rstb.2023.0416. Epub 2024 Sep 16. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39278254 Review. - The effects of shared, depression-specific, and anxiety-specific internalizing symptoms on negative and neutral episodic memories following post-learning sleep.
Niu X, Utayde MF, Sanders KEG, Cunningham TJ, Zhang G, Kensinger EA, Payne JD. Niu X, et al. Cogn Affect Behav Neurosci. 2024 Aug 13. doi: 10.3758/s13415-024-01209-5. Online ahead of print. Cogn Affect Behav Neurosci. 2024. PMID: 39138784 - Targeted Memory Reactivation during Nonrapid Eye Movement Sleep Enhances Neutral, But Not Negative, Components of Memory.
Denis D, Payne JD. Denis D, et al. eNeuro. 2024 May 30;11(5):ENEURO.0285-23.2024. doi: 10.1523/ENEURO.0285-23.2024. Print 2024 May. eNeuro. 2024. PMID: 38769012 Free PMC article. - Evidence of an active role of dreaming in emotional memory processing shows that we dream to forget.
Zhang J, Pena A, Delano N, Sattari N, Shuster AE, Baker FC, Simon K, Mednick SC. Zhang J, et al. Sci Rep. 2024 Apr 15;14(1):8722. doi: 10.1038/s41598-024-58170-z. Sci Rep. 2024. PMID: 38622204 Free PMC article.
References
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. - PubMed
- Ellenbogen JM, Hulbert JC, et al. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Current Biology. 2006;16:1290–4. - PubMed
- Garoff RJ, Slotnick SD, Schacter DL. The neural origins of specific and general memory: The role of the fusiform cortex. Neuropsychologia. 2005;43:847–859. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources