Improving NMR protein structure quality by Rosetta refinement: a molecular replacement study - PubMed (original) (raw)
Improving NMR protein structure quality by Rosetta refinement: a molecular replacement study
Theresa A Ramelot et al. Proteins. 2009 Apr.
Abstract
The structure of human protein HSPC034 has been determined by both solution nuclear magnetic resonance (NMR) spectroscopy and X-ray crystallography. Refinement of the NMR structure ensemble, using a Rosetta protocol in the absence of NMR restraints, resulted in significant improvements not only in structure quality, but also in molecular replacement (MR) performance with the raw X-ray diffraction data using MOLREP and Phaser. This method has recently been shown to be generally applicable with improved MR performance demonstrated for eight NMR structures refined using Rosetta (Qian et al., Nature 2007;450:259-264). Additionally, NMR structures of HSPC034 calculated by standard methods that include NMR restraints have improvements in the RMSD to the crystal structure and MR performance in the order DYANA, CYANA, XPLOR-NIH, and CNS with explicit water refinement (CNSw). Further Rosetta refinement of the CNSw structures, perhaps due to more thorough conformational sampling and/or a superior force field, was capable of finding alternative low energy protein conformations that were equally consistent with the NMR data according to the Recall, Precision, and F-measure (RPF) scores. On further examination, the additional MR-performance shortfall for NMR refined structures as compared with the X-ray structure were attributed, in part, to crystal-packing effects, real structural differences, and inferior hydrogen bonding in the NMR structures. A good correlation between a decrease in the number of buried unsatisfied hydrogen-bond donors and improved MR performance demonstrates the importance of hydrogen-bond terms in the force field for improving NMR structures. The superior hydrogen-bond network in Rosetta-refined structures demonstrates that correct identification of hydrogen bonds should be a critical goal of NMR structure refinement. Inclusion of nonbivalent hydrogen bonds identified from Rosetta structures as additional restraints in the structure calculation results in NMR structures with improved MR performance.
(c) 2008 Wiley-Liss, Inc.
Figures
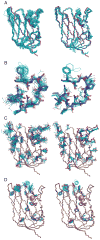
Figure 1
Structure of HSPC034, (A) Secondary structure superimposed on sequence (adapted from PDBsum,
http://www.ebi.ac.uk/thornton-srv/databases/pdbsum/
). Residues that coordinate metal ions are marked with a blue dot. (B) Ribbon representation of X-ray structure of HSPC034 for deposited coordinates, residues 4–139. The Calcium ion is shown in yellow and a Samarium (III) ion in green. (C) Backbone atoms for 20 NMR structures optimally superimposed with respect the N, Cα, and C′ coordinates of the X-ray structure residues 6–138. NMR residues 2–141 are shown. (D) NOE violations indicated on X-ray structure. Red violations are > 2 Å, orange are 1–2 Å, and yellow are 0.5–1 Å. Violations are not show for residues 1–3 and 140–141. Figures B, C, and D were generated using PyMOL (DeLano Scientific).
Figure 2
Comparison of CNSw refined NMR structures (left) to Rosetta refined NMR structures (right). The ensemble of 20 structures is shown as lines for (A) backbone atoms, (B) calcium-binding loop, residues 28–38, (C) side chains for residues DNEQ, and (E) side chains for residues WYF. In all cases, the structures are superimposed on the X-ray structure shown with thick lines.
Figure 3
A) Rosetta all atom energy versus backbone RMSD (residues 4–139) for NMR Rosetta refined structures (squares), and X-ray Rosetta refined structures with parallel (circles) and antiparallel (triangles) β1-strand structures.
Figure 4
(A) Residues that have nearby symmetry related molecules in the X-ray structure are marked with an x for backbone-backbone (bb-bb), backbone-sidechain (bb-sc) and sidechain-sidechain (sc-sc) close contact less than 5 Å. The secondary structure schematic with the metal coordinating residues marked with a filled circle shown above. (B) Backbone RMSD per residue for idealized X-ray structure vs. the X-ray structure after backbone superposition for residues 6–138 for Xros-para, (C) for Xros-anti (D) for Rosetta refined CNSw NMR structures.
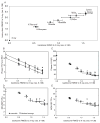
Figure 5
(A) Backbone RMSD (x-axis) versus heavy atom RMSD (y-axis) for residues 6–138. (B–F) Molecular replacement solution metrics (y-axis) versus backbone RMSD for residues 6–138 (x-axis) for Phaser (B) TFZ, triangles, and RFZ, circles, (C) LLG (R) squares, MOLREP (D) RF/s and (E) TF/s. From left to right, the structures are: X-ray, XRos-anti, XRos-para, Rosetta, CNSwRosHB (+RosHBs), CNSw, Xplor, Cyana, Dyana represented with circles. All error bars are one standard deviation for the ensemble. Idealized-average structures for the ensemble are shown with open symbols or Xs. For the X-ray structure, the idealized structure is indicated. Error bars are one standard deviation for the ensembles and are not shown in (B) for the x-axis for clarity.
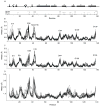
Figure 6
A) Average number of unsatisfied hydrogen bond donors (open circles) and acceptors (open triangles) versus the backbone RMSD to the X-ray structure (residues 6-138) for each ensemble of 20 structures as in Figure 2. (B) Phaser LLG (RF) (filled squares) and LLG (TF) (open circles) vs. the average number of unsatisfied hydrogen bond donors in each ensemble of 20 structures.
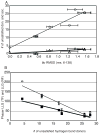
Figure 7
(A) Percent coincidence for all hydrogen bonds (All), filtered to remove bivalent hydrogen bonds (Filtered), and long-range (| j | > 5) filtered hydrogen bonds (Filt long). Coincidence is (number in both)/(number in X-ray only + number in NMR only + both). (B) Counts of hydrogen bonds in each ensemble of 20 structures (1 structure for X-ray) for all hydrogen bonds, filtered to remove bivalent hydrogen bonds, all long range (HNi to COi−j, | j | > 5) (, i+2 (j = 2), and i+3 (j = 3) hydrogen bonds.
Similar articles
- Protein NMR structures refined with Rosetta have higher accuracy relative to corresponding X-ray crystal structures.
Mao B, Tejero R, Baker D, Montelione GT. Mao B, et al. J Am Chem Soc. 2014 Feb 5;136(5):1893-906. doi: 10.1021/ja409845w. Epub 2014 Jan 23. J Am Chem Soc. 2014. PMID: 24392845 Free PMC article. - Comparison of NMR and crystal structures of membrane proteins and computational refinement to improve model quality.
Koehler Leman J, D'Avino AR, Bhatnagar Y, Gray JJ. Koehler Leman J, et al. Proteins. 2018 Jan;86(1):57-74. doi: 10.1002/prot.25402. Epub 2017 Nov 8. Proteins. 2018. PMID: 29044728 Free PMC article. - Refinement of NMR structures using implicit solvent and advanced sampling techniques.
Chen J, Im W, Brooks CL 3rd. Chen J, et al. J Am Chem Soc. 2004 Dec 15;126(49):16038-47. doi: 10.1021/ja047624f. J Am Chem Soc. 2004. PMID: 15584737 - How to tackle protein structural data from solution and solid state: An integrated approach.
Carlon A, Ravera E, Andrałojć W, Parigi G, Murshudov GN, Luchinat C. Carlon A, et al. Prog Nucl Magn Reson Spectrosc. 2016 Feb;92-93:54-70. doi: 10.1016/j.pnmrs.2016.01.001. Epub 2016 Jan 21. Prog Nucl Magn Reson Spectrosc. 2016. PMID: 26952192 Review. - Recent Advances in NMR Protein Structure Prediction with ROSETTA.
Koehler Leman J, Künze G. Koehler Leman J, et al. Int J Mol Sci. 2023 Apr 25;24(9):7835. doi: 10.3390/ijms24097835. Int J Mol Sci. 2023. PMID: 37175539 Free PMC article. Review.
Cited by
- Assessing the chemical accuracy of protein structures via peptide acidity.
Anderson JS, Hernández G, LeMaster DM. Anderson JS, et al. Biophys Chem. 2013 Jan;171:63-75. doi: 10.1016/j.bpc.2012.10.005. Epub 2012 Nov 2. Biophys Chem. 2013. PMID: 23182463 Free PMC article. - MolProbity for the masses-of data.
Chen VB, Wedell JR, Wenger RK, Ulrich EL, Markley JL. Chen VB, et al. J Biomol NMR. 2015 Sep;63(1):77-83. doi: 10.1007/s10858-015-9969-9. Epub 2015 Jul 21. J Biomol NMR. 2015. PMID: 26195077 Free PMC article. - Improved crystallographic models through iterated local density-guided model deformation and reciprocal-space refinement.
Terwilliger TC, Read RJ, Adams PD, Brunger AT, Afonine PV, Grosse-Kunstleve RW, Hung LW. Terwilliger TC, et al. Acta Crystallogr D Biol Crystallogr. 2012 Jul;68(Pt 7):861-70. doi: 10.1107/S0907444912015636. Epub 2012 Jun 19. Acta Crystallogr D Biol Crystallogr. 2012. PMID: 22751672 Free PMC article. - PDBStat: a universal restraint converter and restraint analysis software package for protein NMR.
Tejero R, Snyder D, Mao B, Aramini JM, Montelione GT. Tejero R, et al. J Biomol NMR. 2013 Aug;56(4):337-51. doi: 10.1007/s10858-013-9753-7. Epub 2013 Jul 30. J Biomol NMR. 2013. PMID: 23897031 Free PMC article. - Collective variable approaches for single molecule flexible fitting and enhanced sampling.
Vashisth H, Skiniotis G, Brooks CL 3rd. Vashisth H, et al. Chem Rev. 2014 Mar 26;114(6):3353-65. doi: 10.1021/cr4005988. Epub 2014 Jan 21. Chem Rev. 2014. PMID: 24446720 Free PMC article. Review. No abstract available.
References
- Rossman MG, Blow DM. The detection of sub-units within the crystallographic asymmetric unit. Acta Crystallogr A. 1962;15:24–31.
- Rossman MG. The molecular replacement method. New York, NY: Gordon and Breach, Science Publishers, Inc; 1972.
- Brunger AT, Campbell RL, Clore GM, Gronenborn AM, Karplus M, Petsko GA, Teeter MM. Solution of a protein crystal structure with a model obtained from NMR interproton distance restraints. Science. 1987;235:1049–1053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases