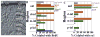The developmental sequence of gene expression within the rod photoreceptor lineage in embryonic zebrafish - PubMed (original) (raw)
The developmental sequence of gene expression within the rod photoreceptor lineage in embryonic zebrafish
Steve M Nelson et al. Dev Dyn. 2008 Oct.
Abstract
In postembryonic zebrafish, rod photoreceptors are continuously generated from progenitors in the inner nuclear layer, which are derived from radial Müller glia that express the transcription factor pax6. We used BrdU incorporation, in combination with in situ hybridization for cell-specific transcription factors, to establish the patterns of gene expression during rod lineage maturation in the embryonic zebrafish. Downregulation of pax6 expression was accompanied by sporadic upregulation of expression of the transcription factors NeuroD/nrd, rx1, crx, and Nr2e3/pnr. As cells of the rod lineage entered the outer nuclear layer, they became homogeneous, coordinately expressing NeuroD, rx1, crx, and Nr2e3. Postmitotic, maturing rods also expressed nrl, rod opsin, and rod transducin/gnat1. The presence of rx1 within the rod lineage and in maturing rods indicates that rx1 is not cone-specific, as previously reported, and suggests a high degree of molecular similarity between rod and cone progenitor populations in the zebrafish.
Copyright (c) 2008 Wiley-Liss, Inc.
Figures
Fig. 1
BrdU incorporation within the rod lineage in embryonic zebrafish. Embryos were injected with BrdU at 60 hpf and fixed at 75 hpf. A: Retinal cryosection showing distribution of BrdU in the circumferential germinal zone (cgz) and in radial arrays of cells (arrowhead) spanning the inner (inl) and outer nuclear layers (onl) in central retina; le, lens; rpe, retinal pigmented epithelium; gc, ganglion cell layer; v, ventral; d, dorsal. B,C: BrdU and rod opsin; Nomarski optics (B) merged with epifluorescent image (C); arrow indicates colabeled cell. D: BrdU and gnat2 colabeling showing BrdU-labeled cones in retinal periphery (arrow) and cells in the central onl labeled only for BrdU (arrowheads). Regions to the periphery of the thin white lines (located approximately 6 onl cell diameters from the unlaminated cgz) were excluded from subsequent analyses related to the rod lineage. E–G: BrdU and anti-GS; arrow indicates colabeled cell. H–J: BrdU and islet1; no colabeling was observed (arrowhead indicates singly-labeled cell). K–M: Section doubly-labeled for BrdU and PKC; no colabeling was observed (arrowhead indicates singly-labeled cell). Scale bar in A (applies to A, D) = 40 μm, in B (applies to B,C,E-M) = 10 μm.
Fig. 2
BrdU labeling kinetics, proliferation kinetics, and rod and cone colabeling in zebrafish embryos. A: Embryos were injected with BrdU and fixed at the indicated times, with low or high doses of BrdU, and sections stained for BrdU indirect immunofluorescence were analyzed for numbers of BrdU+ cells in the inner (inl) and outer nuclear layers (onl). *Significant difference (ANOVA + Fisher’s post-hoc; P < 0.01). B: Sections were stained for PH3 indirect immunofluorescence, and numbers of PH3+ cells in the inl and onl were counted. *Significant difference (ANOVA + Fisher’s post-hoc; P < 0.01). C: Embryos were injected with BrdU at the indicated times, with low or high doses of BrdU, and sections stained for BrdU in combination with in situ hybridization for rod opsin were analyzed for proportion of rod opsin+ cells colabeled with BrdU. *Significant difference (Kolmogorov-Smirnov; P < 0.01). D: Embryos were injected with BrdU and fixed at the indicated times, with low or high doses of BrdU, and sections were stained for BrdU and zpr-1 indirect immunofluorescence, or for BrdU in combination with in situ hybridization for gnat2, then analyzed for numbers of colabeled cones in dorsal and ventral retina.
Fig. 3
Gene expression in cells of the BrdU+ rod lineage. All embryos were injected with BrdU at 60 hpf and fixed at 75 or 80 hpf. A,B: BrdU and pax6; Nomarski optics (A) merged with epifluorescence image (B); arrow indicates colabeled cell. C,D: BrdU and NeuroD; Nomarski optics (C), merged with epifluorescence image (D); two colabeled cells are indicated by arrows. E,F: BrdU and crx; Nomarski optics (E), merged with epifluorescence image (F); arrow indicates colabeled cell. G–J: BrdU and Nr2e3; Nomarski optics (G,I), merged with epifluorescence images (H,J); colabeled cells are indicated by arrows. K: Retinal expression pattern of nrl; both rod (arrow) and cone progenitors (arrowhead) are labeled, as well as cells of the lens (*). L,M: BrdU and nrl; Nomarski optics (L), merged with epifluorescence image (M); arrow indicates colabeled cell. N,O: BrdU and rx1; Nomarski optics (N), merged with epifluorescence image (O); colabeled cells are indicated by arrows. rpe, retinal pigmented epithelium; onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer; le, lens. Scale bar in A (applies to all except K) = 10 μm, in K = 40 μm.
Fig. 4
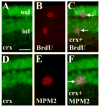
Crx protein expression in mitotic cells of the BrdU+ rod lineage. A–C: Embryo was injected with BrdU at 60 hpf and fixed at 75 hpf, and processed for indirect immunofluorescence with anti-crx (A) and anti-BrdU (B). C shows merged image and colabeled cells (arrows). D–F: Embryo fixed at 72 hpf and processed for indirect immunofluorescence with anti-crx (D) and MPM-2 (mitotic cells; E). F shows merged image and colabeled cells (arrow). Scale bar in A (applies to all) = 10 μm.
Fig. 5
Colabeling quantification by onl and inl compartments. A: Retinal cryosection from 72-hpf embryo showing subdivision of the inner nuclear layer (inl) into proximal (p-inl), medial (m-inl), and distal (d-inl) compartments; further explanation is provided in the Results section. B: BrdU colabeling with rod opsin, nrl, Nr2e3, crx, rx1, NeuroD, and pax6, by retinal lamina and defined inl compartments. C: Rx1 colabeling with rod opsin, crx, NeuroD, and pax6, by retinal lamina and defined compartments of the inl. rpe, retinal pigmented epithelium; onl, outer nuclear layer; gcl, ganglion cell layer.
Fig. 6
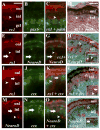
Rx1 expression in all photoreceptor subtypes. All panels show retinal cryosections of 72-hpf embryos. A–C: rx1 (A) and rod opsin (B) colabeling (C). D–F: rx1 (D) and gnat1 (E) colabeling (F). G–I: rod opsin (G) and gnat1 (H) colabeling (I; positive control). J–L: rod opsin (J) and gnat2 (K) show no colabeling (L; negative control). M,N: rx1 and rod opsin colabeling using an alternative set of reaction products; N is an enlargement of region in M indicated by *, showing colabeled cells. O–Q: rx1 (O) and red opsin (P) colabeling (Q). R–T: rx1 (R) and blue opsin (S) colabeling (T). U–W: rx1 (U) and uv opsin (V) colabeling (W). X–Z: rx1 (X) and gnat2 (Y) colabeling (Z). onl, outer nuclear layer; inl, inner nuclear layer; gcl, ganglion cell layer; rpe, retinal pigmented epithelium. Scale bar in A (applies to A–L, O–W) = 20 μm; in M = 50 μm.
Fig. 7
Rx1 coexpression with rod lineage markers. A–C: rx1 (A) and pax6 (B) double in situ; merged image (C) showing no colabeled cells (arrowhead). D: rx1 (purple) and pax6 (pink) using alternative reaction products; arrowhead = _rx1_+ cell only. E–G: rx1 (E) and NeuroD (F); merged image (G) showing colabeling in the inl (arrow) and throughout the onl; arrowhead = _rx1_+ cell only. H: rx1 (purple) and NeuroD (pink) using alternative reaction products; doubly-labeled cell (arrow) and _rx1_+ cell only (arrowhead). I–K: rx1 (I) and crx (J); merged image (K) showing a colabeled cell in the inl (arrow) and throughout the onl; arrowhead = _rx1_+ cell only. L: rx1 (purple) and crx (pink) using alternative reaction products, showing a doubly-labeled cell (arrow) and an _rx1_+ cell only (arrowhead). M–O: NeuroD (M) and crx (N); merged image (O) showing a colabeled cell in the inl (arrow) and throughout the onl; arrowhead = _NeuroD_+ cell only. P: NeuroD (pink) and crx (purple) using alternative reaction products, showing doubly-labeled cell (arrow). onl, outer nuclear layer; inl, inner nuclear layer; scale bar in A (applies to A–C,E–G,I–K,M–O) = 20 μm; in D (applies to E,H,L,P) = 20 μm. *, areas enlarged in insets.
Fig. 8
Gene expression in the embryonic rod lineage of zebrafish. Gene expression summary at right shows the genetic hierarchy of the rod lineage; darkness of font reflects the extent to which the transcription factor is expressed within the identified cells of the lineage. _Pax6_-positive Müller glia/stem cells are shown in blue with their cell bodies in the inner region of the inl. The summary at right illustrates an alternative scenario in which _pax6_+ stem cells (in black) generate embryonic Müller glia as well as cells of the rod lineage. As rod progenitors proliferate and migrate toward the onl, some become _NeuroD-_positive and _rx1_-positive (green cells), but none remain _pax6-_positive. All of these progenitors later express crx (yellow). Prior to entering the onl, some rod progenitors express NeuroD, rx1, and Nr2e3, while all continue to express crx (orange). All rod precursors of the onl (red) are NeuroD, rx1, crx, and _Nr2e3_-positive but do not express nrl, rod opsin, or gnat 1. Postmitotic rod photoreceptors (purple) express NeuroD, rx1, crx, Nr2e3, nrl, rod opsin, and gnat1. onl, outer nuclear layer; p-inl, m-inl, d-inl are proximal, middle, and distal compartments of the inl, respectively, and are delineated by dotted lines.
Similar articles
- NeuroD regulates proliferation of photoreceptor progenitors in the retina of the zebrafish.
Ochocinska MJ, Hitchcock PF. Ochocinska MJ, et al. Mech Dev. 2009 Mar-Apr;126(3-4):128-41. doi: 10.1016/j.mod.2008.11.009. Epub 2008 Dec 14. Mech Dev. 2009. PMID: 19121642 Free PMC article. - Knockout of Nr2e3 prevents rod photoreceptor differentiation and leads to selective L-/M-cone photoreceptor degeneration in zebrafish.
Xie S, Han S, Qu Z, Liu F, Li J, Yu S, Reilly J, Tu J, Liu X, Lu Z, Hu X, Yimer TA, Qin Y, Huang Y, Lv Y, Jiang T, Shu X, Tang Z, Jia H, Wong F, Liu M. Xie S, et al. Biochim Biophys Acta Mol Basis Dis. 2019 Jun 1;1865(6):1273-1283. doi: 10.1016/j.bbadis.2019.01.022. Epub 2019 Jan 23. Biochim Biophys Acta Mol Basis Dis. 2019. PMID: 30684641 - The rod photoreceptor lineage of teleost fish.
Stenkamp DL. Stenkamp DL. Prog Retin Eye Res. 2011 Nov;30(6):395-404. doi: 10.1016/j.preteyeres.2011.06.004. Epub 2011 Jun 30. Prog Retin Eye Res. 2011. PMID: 21742053 Free PMC article. Review. - Regulation of photoreceptor gene expression by Crx-associated transcription factor network.
Hennig AK, Peng GH, Chen S. Hennig AK, et al. Brain Res. 2008 Feb 4;1192:114-33. doi: 10.1016/j.brainres.2007.06.036. Epub 2007 Jun 30. Brain Res. 2008. PMID: 17662965 Free PMC article. Review.
Cited by
- Recruitment of Rod Photoreceptors from Short-Wavelength-Sensitive Cones during the Evolution of Nocturnal Vision in Mammals.
Kim JW, Yang HJ, Oel AP, Brooks MJ, Jia L, Plachetzki DC, Li W, Allison WT, Swaroop A. Kim JW, et al. Dev Cell. 2016 Jun 20;37(6):520-32. doi: 10.1016/j.devcel.2016.05.023. Dev Cell. 2016. PMID: 27326930 Free PMC article. - Leveraging Zebrafish to Study Retinal Degenerations.
Angueyra JM, Kindt KS. Angueyra JM, et al. Front Cell Dev Biol. 2018 Sep 19;6:110. doi: 10.3389/fcell.2018.00110. eCollection 2018. Front Cell Dev Biol. 2018. PMID: 30283779 Free PMC article. Review. - Nrl Is Dispensable for Specification of Rod Photoreceptors in Adult Zebrafish Despite Its Deeply Conserved Requirement Earlier in Ontogeny.
Oel AP, Neil GJ, Dong EM, Balay SD, Collett K, Allison WT. Oel AP, et al. iScience. 2020 Nov 15;23(12):101805. doi: 10.1016/j.isci.2020.101805. eCollection 2020 Dec 18. iScience. 2020. PMID: 33299975 Free PMC article. - Developing and adult reef fish show rapid light-induced plasticity in their visual system.
Fogg LG, Cortesi F, Gache C, Lecchini D, Marshall NJ, de Busserolles F. Fogg LG, et al. Mol Ecol. 2023 Jan;32(1):167-181. doi: 10.1111/mec.16744. Epub 2022 Nov 11. Mol Ecol. 2023. PMID: 36261875 Free PMC article. - Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish.
Lenkowski JR, Raymond PA. Lenkowski JR, et al. Prog Retin Eye Res. 2014 May;40:94-123. doi: 10.1016/j.preteyeres.2013.12.007. Epub 2014 Jan 8. Prog Retin Eye Res. 2014. PMID: 24412518 Free PMC article. Review.
References
- Akimoto M, Cheng H, Zhu D, Brzezinski JA, Khanna R, Filippova E, Oh EC, Jing Y, Linares JL, Brooks M, Zareparsi S, Mears AJ, Hero A, Glaser T, Swaroop A. Targeting of GFP to newborn rods by Nrl promoter and temporal expression profiling of flow-sorted photoreceptors. Proc Natl Acad Sci U S A. 2006;103:3890–3895. - PMC - PubMed
- Altshuler D, Turner D, Cepko C. Specification of cell types in the vertebrate retina. In: Lam D, Shatz C, editors. Development of the visual system. Cambridge: MIT Press; 1991. pp. 37–58.
- Andreazzoli M, Gestri G, Cremisi F, Casarosa S, Dawid IB, Barsacchi G. Xrx1 controls proliferation and neurogenesis in Xenopus anterior neural plate. Development. 2003;130:5143–5154. - PubMed
- Bailey TJ, El-Hodiri H, Zhang L, Shah R, Mathers PH, Jamrich M. Regulation of vertebrate eye development by Rx genes. Int J Dev Biol. 2004;48:761–770. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01EY012146/EY/NEI NIH HHS/United States
- R29 EY012146/EY/NEI NIH HHS/United States
- P20 RR015587/RR/NCRR NIH HHS/United States
- P20RR015587/RR/NCRR NIH HHS/United States
- R01 EY012146/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials