Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats - PubMed (original) (raw)
Review
Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats
Michelle Longmire et al. Nanomedicine (Lond). 2008 Oct.
Abstract
Nanoparticles possess enormous potential as diagnostic imaging agents and hold promise for the development of multimodality agents with both imaging and therapeutic capabilities. Yet, some of the most promising nanoparticles demonstrate prolonged tissue retention and contain heavy metals. This presents serious concerns for toxicity. The creation of nanoparticles with optimal clearance characteristics will minimize toxicity risks by reducing the duration of exposure to these agents. Given that many nanoparticles possess easily modifiable surface and interior chemistry, if nanoparticle characteristics associated with optimal clearance from the body were well established, it would be feasible to design and create agents with more favorable clearance properties. This article presents a thorough discussion of the physiologic aspects of nanoparticle clearance, focusing on renal mechanisms, and provides an overview of current research investigating clearance of specific types of nanoparticles and nano-sized macromolecules, including dendrimers, quantum dots, liposomes and carbon, gold and silica-based nanoparticles.
Figures
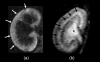
Figure 1
2D-T1 weighted MR images of kidneys with acute tubular dysfunction (a) and normal function (b) are shown. Glomerular filtration (white arrows) is shown in the kidney when the tubular function is completely impaired (a) and tubular concentration function (black arrows) and urinary excretion (*) are shown in the normal functioning kidney (b).
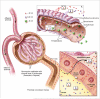
Figure 2
Renal handling of nanoparticles of different sizes and charges. Circulating nanoparticles enter the glomerular capillary bed via the afferent arteriole. A) The glomerular capillary wall is composed of three layers: fenestrated endothelium; the highly negatively charged glomerular basement membrane (GBM); and podocyte extensions of glomerular epithelial cells. Glomerular filtrate flows through the fenestrate, across the GBM, and through filtration slits formed by spaces between podocyte extensions. The primary size barrier is the filtration slit, which has a physiologic pore size of 4.5-5 nm. Nanoparticles < 6 nm (red) are small enough to be freely filtered, irrespectively of molecular charge. However, glomerular filtration of particles between 6-8 nm (purple) is dependent on charge interactions between the intermediate sized particles and the negative charges of the GBM. Therefore, positively particles are more readily filtered than equally sized negatively charged particles. Due to size limitations, particles > 8 nm do not undergo glomerular filtration. After glomerular filtration, filtered nanoparticles enter the proximal tubule. B) Within the proximal tubule, nanoparticles may be resorbed from the luminal space. Since the brush border of the proximal tubule epithelial cells is negatively charged, positively charged particles are more readily resorbed than comparable negatively charged particles.
Figure 3
3D-maximum intensity projection display of T1-weighted MR images of the abdomen at 15 min post-injection for G9, G8, G7, and G6 and at 3 min after injection for G5, G4, G3, G2, and DTPA are shown. Kidneys are shown with agents of G5 (8 nm in diameter) or smaller. Blood pool is clearly depicted G5 (8 nm) and G4 (5 nm) at 3 min post-injection because of partial and slow glomerular filtration. Kidneys are not shown with agents G6 (9 nm in diameter) or larger even at 15 min post-injection. Intensities in the liver increase as the agent sizes increase.
Figure 4
Comparison of sizes of various nanoparticles. Chemical composition and size range are defining features for nanoparticles. Both characteristics are important determinants of nanoparticle in vivo behavior and clearance properties.
Similar articles
- Application of gold nanoparticles in biomedical and drug delivery.
Daraee H, Eatemadi A, Abbasi E, Fekri Aval S, Kouhi M, Akbarzadeh A. Daraee H, et al. Artif Cells Nanomed Biotechnol. 2016;44(1):410-22. doi: 10.3109/21691401.2014.955107. Epub 2014 Sep 17. Artif Cells Nanomed Biotechnol. 2016. PMID: 25229833 Review. - Nanoparticulate assemblies of amphiphiles and diagnostically active materials for multimodality imaging.
Mulder WJ, Strijkers GJ, van Tilborg GA, Cormode DP, Fayad ZA, Nicolay K. Mulder WJ, et al. Acc Chem Res. 2009 Jul 21;42(7):904-14. doi: 10.1021/ar800223c. Acc Chem Res. 2009. PMID: 19435319 Free PMC article. - Engineered nonviral nanocarriers for intracellular gene delivery applications.
Ojea-Jiménez I, Tort O, Lorenzo J, Puntes VF. Ojea-Jiménez I, et al. Biomed Mater. 2012 Oct;7(5):054106. doi: 10.1088/1748-6041/7/5/054106. Epub 2012 Sep 12. Biomed Mater. 2012. PMID: 22972254 - Chemical nature and structure of organic coating of quantum dots is crucial for their application in imaging diagnostics.
Bakalova R, Zhelev Z, Kokuryo D, Spasov L, Aoki I, Saga T. Bakalova R, et al. Int J Nanomedicine. 2011;6:1719-32. doi: 10.2147/IJN.S17995. Epub 2011 Aug 18. Int J Nanomedicine. 2011. PMID: 21980235 Free PMC article. - Carbon nanotubes for biomedical imaging: the recent advances.
Gong H, Peng R, Liu Z. Gong H, et al. Adv Drug Deliv Rev. 2013 Dec;65(15):1951-63. doi: 10.1016/j.addr.2013.10.002. Epub 2013 Oct 30. Adv Drug Deliv Rev. 2013. PMID: 24184130 Review.
Cited by
- In vivo ZW800-microbead imaging of retinal and choroidal vascular leakage in mice.
Gupta I, Cahoon J, Zhang X, Jones AD, Ahmed F, Uehara H, Messenger W, Ambati BK. Gupta I, et al. Exp Eye Res. 2015 May;134:155-8. doi: 10.1016/j.exer.2014.12.013. Epub 2014 Dec 20. Exp Eye Res. 2015. PMID: 25536533 Free PMC article. - The golden age: gold nanoparticles for biomedicine.
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. Dreaden EC, et al. Chem Soc Rev. 2012 Apr 7;41(7):2740-79. doi: 10.1039/c1cs15237h. Epub 2011 Nov 22. Chem Soc Rev. 2012. PMID: 22109657 Free PMC article. Review. - Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview.
Mosleh-Shirazi S, Abbasi M, Moaddeli MR, Vaez A, Shafiee M, Kasaee SR, Amani AM, Hatam S. Mosleh-Shirazi S, et al. Nanotheranostics. 2022 Aug 21;6(4):400-423. doi: 10.7150/ntno.74613. eCollection 2022. Nanotheranostics. 2022. PMID: 36051855 Free PMC article. Review. - Magnetic-guided nanocarriers for ionizing/non-ionizing radiation synergistic treatment against triple-negative breast cancer.
Zhou Y, Kou J, Zhang Y, Ma R, Wang Y, Zhang J, Zhang C, Zhan W, Li K, Li X. Zhou Y, et al. Biomed Eng Online. 2024 Jul 13;23(1):67. doi: 10.1186/s12938-024-01263-7. Biomed Eng Online. 2024. PMID: 39003472 Free PMC article. - Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery.
Dang XTT, Kavishka JM, Zhang DX, Pirisinu M, Le MTN. Dang XTT, et al. Cells. 2020 Sep 29;9(10):2191. doi: 10.3390/cells9102191. Cells. 2020. PMID: 33003285 Free PMC article. Review.
References
- Hagens WI, Oomen AG, de Jong WH, Cassee FR, Sips AJ. What do we (need to) know about the kinetic properties of nanoparticles in the body? Regul Toxicol Pharmacol. 2007 Dec;49:217–29. - PubMed
- Ballou B, Ernst LA, Andreko S, Harper T, Fitzpatrick JA, Waggoner AS, Bruchez MP. Sentinel lymph node imaging using quantum dots in mouse tumor models. Bioconjug Chem. 2007 Mar-Apr;18:389–96. - PubMed
- Csortos C, Kolosova I, Verin AD. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am J Physiol Lung Cell Mol Physiol. 2007 Oct;293:L843–54. - PubMed
- Chapman AP, Antoniw P, Spitali M, West S, Stephens S, King DJ. Therapeutic antibody fragments with prolonged in vivo half-lives. Nat Biotechnol. 1999 Aug;17:780–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical