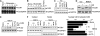Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity - PubMed (original) (raw)
Cdk5 phosphorylates Cdh1 and modulates cyclin B1 stability in excitotoxicity
Carolina Maestre et al. EMBO J. 2008.
Abstract
Anaphase-promoting complex/cyclosome (APC/C), an E3 ubiquitin ligase that destabilizes cell cycle proteins, is activated by Cdh1 in post-mitotic neurons, where it regulates axonal growth, synaptic plasticity and survival. The APC/C-Cdh1 substrate, cyclin B1, has been found to accumulate in degenerating brain areas in Alzheimer's disease and stroke. This highlights the importance of elucidating cyclin B1 regulation by APC/C-Cdh1 in neurons under stress conditions relevant to neurological disease. Here, we report that stimulation of N-methyl-D-aspartate receptors (NMDARs) that occurs in neurodegenerative diseases promoted the accumulation of cyclin B1 in the nuclei of cortical neurons; this led the neurons to undergo apoptotic death. Moreover, we found that the Ser-40, Thr-121 and Ser-163 triple phosphorylation of Cdh1 by the cyclin-dependent kinase-5 (Cdk5)-p25 complex was necessary and sufficient for cyclin B1 stabilization and apoptotic death after NMDAR stimulation. These results reveal Cdh1 as a novel Cdk5 substrate that mediates cyclin B1 neuronal accumulation in excitotoxicity.
Figures
Figure 1
Cyclin B1 accumulation mediates neuronal apoptotic death induced by NMDAR stimulation in cortical neurons. Cortical neurons in primary culture were incubated in the presence of glutamate (100 μM) or NMDA (100 μM) for 5 min; after washing, cells were further incubated in culture medium for 20 h. (A) Analyses of cyclin B1 (phycoerythrin-conjugated) levels by flow cytometry revealed that glutamate induced an ∼6.6-fold increase in cyclin B1. (B) Western blot analysis revealed that glutamate or NMDA promoted cyclin B1 accumulation in the nuclei of neurons. (C) Immunocytochemical evidence for cyclin B1 nuclear localization in neurons by glutamate treatment ( × 100 magnification). (D) Knockdown of cyclin B1 (cyclin B1 shRNA) in neurons prevents the apoptotic death triggered by glutamate or NMDA. *P<0.05 versus none. Topoisomerase-II (TopoII, A) was used as protein loading marker.
Figure 2
Cdh1 modulates cyclin B1 stability after NMDAR stimulation in cortical neurons. (A) Cdh1 shRNA expression induces Cdh1 depletion, as revealed by immunostaining ( × 20 magnification). (B) Cdh1 knockdown sensitizes neurons towards excitotoxic damage, as apoptotic death was accelerated and enhanced by Cdh1 shRNA expression. (C) Cdh1 knockdown synergistically enhanced the proportion of fragmented or condensed nuclei induced by glutamate after 20 h. (D) Cdh1 knockdown enhanced apoptotic death caused by glutamate or NMDA, as assessed by annexin V/7-AAD; the NMDA receptor inhibitor, MK-801, prevented glutamate-mediated apoptotic death. (E) Cyclin B1 shRNA abolished the increased apoptotic death triggered 20 h after glutamate (100 μM/5 min) or NMDA (100 μM/5 min) treatments in Cdh1-silenced neurons. *P<0.05 versus none.
Figure 3
NMDAR stimulation produces serine phosphorylation and accumulation of Cdh1 in the cytosol. (A) Northern blot analysis revealed that glutamate (100 μM/5 min) did not alter Cdh1 mRNA abundance, at least up to 24 h. (B) Glutamate did not change Cdh1 protein abundance, but it caused Cdh1 mobility super-shift in the gel. (C) Cdh1 mobility super-shift at 4 h after glutamate incubation was abolished by NMDAR inhibition (using MK-801) or by removing calcium (using EDTA or BAPTA), and it was mimicked by NMDA. (D) Protein phosphatase (λpp) treatment of samples obtained from neurons at 4 h after glutamate or NMDA stimulation prevents Cdh1 mobility super-shift. (E) Protein extracts were obtained from NMDA (100 μM/5 min)-treated neurons at the indicated time points, Cdh1 was immunoprecipitated and subjected to western blotting against α-phosphoserine (P-Ser); NMDA time-dependently triggered Ser phosphorylation of Cdh1 that was prevented by removing calcium (using EDTA or BAPTA). (F) NMDA induced Cdh1 accumulation in the cytosol and depletion in the nuclei in a time-dependent manner; both effects were prevented by calcium removal (using EDTA or BAPTA); topoisomerase-II (TopoII) was undetectable in the cytosol (left panel), and GAPDH was negligibly expressed in the nuclei (middle panel); films (left and middle panels) were scanned and the nuclear/cytosolic Cdh1 band intensity ratios were calculated (right panel). *P<0.05 versus none. Cyclophilin was used as RNA loading marker, and GAPDH (B, C, D, F), Cdh1 (E) or topoisomerase-II (TopoII, F) were used as protein loading markers.
Figure 4
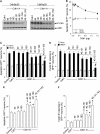
NMDAR stimulation activates Cdk5–p25, which interacts with, and phosphorylates Cdh1, leading to cyclin B1 accumulation and apoptotic death. (A) In vitro Cdk5 kinase assay showed that both Cdk5–p35 and Cdk5–p25 complexes phosphorylate Cdh1, Cdk5–p25 being about three orders of magnitude more efficient than Cdk5–p35. (B) NMDA (100 μM/5 min) time-dependently triggered p35 decrease and p25 accumulation in cortical neurons. (C) NMDAR stimulation triggers Cdk5 activation as assayed by histone 1 (H1) phosphorylation in vitro. (D) Neurons were incubated with glutamate or NMDA; after 4 h, protein extracts were immunoprecipitated with α-Cdk5 and subjected to western blotting against α-Cdk5 and α-Cdh1; glutamate and NMDA increased Cdh1 band intensity. (E) NMDA treatment produced Cdh1 mobility super-shift that was prevented by Cdk5 knockdown using Cdk5 siRNA. (F) Incubation of protein extracts with protein phosphatase (λpp) decreased the Cdh1 mobility super-shift caused by NMDA in control siRNA-treated neurons; this effect was not evident in Cdk5 siRNA-treated neurons. (G) Cdk5 siRNA dose-dependently prevented the serine phosphorylation of Cdh1 induced 4 h after NMDA treatment. (H) Cdk5 siRNA abolished the increase in cyclin B1 caused by NMDA treatment, as assessed by flow cytometry after 20 h using a cyclin B1-phycoerythrin (PE) antibody. (I) Cdk5 siRNA dose-dependently prevented glutamate- and NMDA-mediated apoptotic death, as assessed by flow cytometry using annexin V/7-ADD (20 h post-treatment). (J) Inhibition of APC/C–Cdh1 (using hEmi1 expression) or cyclin B1 overexpression counteracted the protective effect caused by Cdk5 siRNA on NMDA-mediated neuronal apoptotic death. (K) MDL, a specific inhibitor of calpain, dose-dependently prevented NMDA-mediated p35 cleavage into p25. MDL prevented (L) Cdk5 activation, (M) Cdh1 gel shift and (N) cyclin B1 accumulation caused by NMDAR stimulation. *P<0.05 versus none. #P<0.05 versus Cdk5 siRNA+NMDA (in J). GAPDH (B, E, F, K, N) was used as protein loading marker.
Figure 5
Triple phosphorylation of Cdh1 at Ser-40, Thr-121 and Ser-163 by Cdk5 is necessary and sufficient for cyclin B1 accumulation and excitotoxicity. Residues Ser-40, Thr-121 and Ser-163 of Cdh1 were mutated to either Ala (Cdh1-A) or Asp (Cdh1-D) by site-directed mutagenesis as single, double or triple mutations. Both the wild-type and all Ala-mutant forms of Cdh1 were expressed and affinity purified. (A) The in vitro kinase assay on the Ala mutant forms of Cdh1 by Cdk5–p35 (1 μg) and Cdk5–p25 (1 ng) shows that single mutations do not produce observable decrease in Cdh1 phosphorylation, double mutations slightly reduced Cdh1 phosphorylation, and triple mutation abolished Cdh1 phosphorylation. (B) Cdh1 expression in cortical neurons dose-dependently prevented NMDA-mediated apoptotic death, the minimum Cdh1 cDNA amount not altering NMDA-mediated apoptosis being 0.32 μg/106 neurons. This amount was used for the following experiments. (C) NMDA-mediated apoptotic death, as assessed by flow cytometry using annexin V/7-ADD (20 h post-treatment), was not prevented by the expression in neurons of equal amounts of Cdh1 cDNA carrying the single Ala mutations, it was slightly prevented by Cdh1 carrying the double Ala mutations and it was significantly prevented by Cdh1 carrying the triple Ala mutation. (D) NMDA-mediated cyclin B1 accumulation, as assessed by flow cytometry after 20 h using a cyclin B1-phycoerythrin (PE) antibody, was not prevented by the expression in neurons of equal amounts of Cdh1 cDNA carrying the single Ala mutations, it was slightly prevented by Cdh1 carrying the double Ala mutations and it was abolished by Cdh1 carrying the triple Ala mutation. (E) Neuronal apoptotic death, as assessed by flow cytometry using annexin V/7-ADD (20 h post-treatment), was not altered by the expression of equal amounts of Cdh1 cDNA carrying the Asp single mutations, it was significantly increased by Cdh1 carrying the double Asp mutations and it further increased by Cdh1 carrying the triple Asp mutation, reaching values similar to those observed with NMDA treatment (See C). (F) Cyclin B1 accumulation, as assessed by flow cytometry after 20 h using a cyclin B1-PE antibody, was not altered by the expression of equal amounts of Cdh1 cDNA carrying the Asp single mutations, it was significantly increased by Cdh1 carrying the double Asp mutations and it further increased by Cdh1 carrying the triple Asp mutation, reaching values similar to those observed with NMDA treatment (See C). *P<0.05 versus control. #P<0.05 versus control NMDA.
Similar articles
- Cdk5-mediated inhibition of APC/C-Cdh1 switches on the cyclin D1-Cdk4-pRb pathway causing aberrant S-phase entry of postmitotic neurons.
Veas-Pérez de Tudela M, Maestre C, Delgado-Esteban M, Bolaños JP, Almeida A. Veas-Pérez de Tudela M, et al. Sci Rep. 2015 Dec 10;5:18180. doi: 10.1038/srep18180. Sci Rep. 2015. PMID: 26658992 Free PMC article. - Cdh1/Hct1-APC is essential for the survival of postmitotic neurons.
Almeida A, Bolaños JP, Moreno S. Almeida A, et al. J Neurosci. 2005 Sep 7;25(36):8115-21. doi: 10.1523/JNEUROSCI.1143-05.2005. J Neurosci. 2005. PMID: 16148219 Free PMC article. - Regulation of Bcl-xL-ATP Synthase Interaction by Mitochondrial Cyclin B1-Cyclin-Dependent Kinase-1 Determines Neuronal Survival.
Veas-Pérez de Tudela M, Delgado-Esteban M, Maestre C, Bobo-Jiménez V, Jiménez-Blasco D, Vecino R, Bolaños JP, Almeida A. Veas-Pérez de Tudela M, et al. J Neurosci. 2015 Jun 24;35(25):9287-301. doi: 10.1523/JNEUROSCI.4712-14.2015. J Neurosci. 2015. PMID: 26109654 Free PMC article. - Cdh1-APC/C, cyclin B-Cdc2, and Alzheimer's disease pathology.
Aulia S, Tang BL. Aulia S, et al. Biochem Biophys Res Commun. 2006 Jan 6;339(1):1-6. doi: 10.1016/j.bbrc.2005.10.059. Epub 2005 Oct 21. Biochem Biophys Res Commun. 2006. PMID: 16253208 Review. - Brain energy metabolism in glutamate-receptor activation and excitotoxicity: role for APC/C-Cdh1 in the balance glycolysis/pentose phosphate pathway.
Rodriguez-Rodriguez P, Almeida A, Bolaños JP. Rodriguez-Rodriguez P, et al. Neurochem Int. 2013 Apr;62(5):750-6. doi: 10.1016/j.neuint.2013.02.005. Epub 2013 Feb 12. Neurochem Int. 2013. PMID: 23416042 Review.
Cited by
- Fzr/Cdh1 Promotes the Differentiation of Neural Stem Cell Lineages in Drosophila.
Ly PT, Wang H. Ly PT, et al. Front Cell Dev Biol. 2020 Feb 11;8:60. doi: 10.3389/fcell.2020.00060. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32117986 Free PMC article. - Cdk5-mediated inhibition of APC/C-Cdh1 switches on the cyclin D1-Cdk4-pRb pathway causing aberrant S-phase entry of postmitotic neurons.
Veas-Pérez de Tudela M, Maestre C, Delgado-Esteban M, Bolaños JP, Almeida A. Veas-Pérez de Tudela M, et al. Sci Rep. 2015 Dec 10;5:18180. doi: 10.1038/srep18180. Sci Rep. 2015. PMID: 26658992 Free PMC article. - PFKFB3 ameliorates ischemia-induced neuronal damage by reducing reactive oxygen species and inhibiting nuclear translocation of Cdk5.
Kwon HJ, Hahn KR, Moon SM, Yoo DY, Kim DW, Hwang IK. Kwon HJ, et al. Sci Rep. 2024 Oct 21;14(1):24694. doi: 10.1038/s41598-024-75031-x. Sci Rep. 2024. PMID: 39433564 Free PMC article. - Cdk5 levels oscillate during the neuronal cell cycle: Cdh1 ubiquitination triggers proteosome-dependent degradation during S-phase.
Zhang J, Li H, Zhou T, Zhou J, Herrup K. Zhang J, et al. J Biol Chem. 2012 Jul 27;287(31):25985-94. doi: 10.1074/jbc.M112.343152. Epub 2012 May 31. J Biol Chem. 2012. PMID: 22654103 Free PMC article. - A standardized randomized 6-month aerobic exercise-training down-regulated pro-inflammatory genes, but up-regulated anti-inflammatory, neuron survival and axon growth-related genes.
Iyalomhe O, Chen Y, Allard J, Ntekim O, Johnson S, Bond V, Goerlitz D, Li J, Obisesan TO. Iyalomhe O, et al. Exp Gerontol. 2015 Sep;69:159-69. doi: 10.1016/j.exger.2015.05.005. Epub 2015 May 15. Exp Gerontol. 2015. PMID: 25981742 Free PMC article. Clinical Trial.
References
- Almeida A, Bolaños JP (2001) A transient inhibition of mitochondrial ATP synthesis by nitric oxide synthase activation triggered apoptosis in primary cortical neurons. J Neurochem 77: 676–690 - PubMed
- Almeida A, Moncada S, Bolaños JP (2004) Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructo-2-kinase pathway. Nat Cell Biol 6: 45–51 - PubMed
- Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M (2004) Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428: 190–193 - PubMed
- Becker EBE, Bonni A (2004) Cell cycle regulation of neuronal apoptosis in development and disease. Progr Neurobiol 72: 1–25 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous