Regulation of neural progenitor cell development in the nervous system - PubMed (original) (raw)
Review
Regulation of neural progenitor cell development in the nervous system
Joshua G Corbin et al. J Neurochem. 2008 Sep.
Abstract
The mammalian telencephalon, which comprises the cerebral cortex, olfactory bulb, hippocampus, basal ganglia, and amygdala, is the most complex and intricate region of the CNS. It is the seat of all higher brain functions including the storage and retrieval of memories, the integration and processing of sensory and motor information, and the regulation of emotion and drive states. In higher mammals such as humans, the telencephalon also governs our creative impulses, ability to make rational decisions, and plan for the future. Despite its massive complexity, exciting work from a number of groups has begun to unravel the developmental mechanisms for the generation of the diverse neural cell types that form the circuitry of the mature telencephalon. Here, we review our current understanding of four aspects of neural development. We first begin by providing a general overview of the broad developmental mechanisms underlying the generation of neuronal and glial cell diversity in the telencephalon during embryonic development. We then focus on development of the cerebral cortex, the most complex and evolved region of the brain. We review the current state of understanding of progenitor cell diversity within the cortical ventricular zone and then describe how lateral signaling via the Notch-Delta pathway generates specific aspects of neural cell diversity in cortical progenitor pools. Finally, we review the signaling mechanisms required for development, and response to injury, of a specialized group of cortical stem cells, the radial glia, which act both as precursors and as migratory scaffolds for newly generated neurons.
Figures
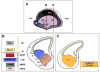
Figure 1. Progenitor domains in the telencephalon
Schematic of a sagittal hemisection of a mid-neurogenesis (approximately E13.5) embryo revealing the subpallial ganglionic eminences in relation to the pallium is shown in (A). Coronal sections at the level of the MGE/LGE and CGE are shown in (B) and (C). Based on a combination of the expression of VZ/SVZ transcription factors, the telencephalon can be subdivided into distinct progenitor domains that generate different cell types as shown in (B) and (C). As shown in (B), the pallium can be subdivided into at least 2 domains; the dorsal pallium (dP), which gives rise to glutamatergic neurons (Glu+) and the ventral pallium which may also give rise to Glu+ neurons. The MGE and LGE can be also further subdivided, with the vMGE giving rise to parvalbuim (PV+) interneurons and the dMGE somatostatin (SOM+) interneurons. In contrast, the body of the LGE is a major source of DARPP32+ inhibitory projection neurons, and the dLGE is a putative source of calretinin (CR+) interneurons. As shown in (C), the CGE is a major source of CR+ and VIP+ interneurons, and DARPP32+ inhibitory projection neurons.
Figure 2. Major routes of interneuron migration in the telencephalon
Major routes of cell migration of immature interneurons are shown in coronal views at the level of the MGE/LGE (A) and CGE (B) in the embryonic telencephalon. The right side of the panel shows actual migratory routes as revealed by β-galactosidase staining of Dlx2tauLacZ transgenic mice in which LacZ was knocked into the Dlx2 locus. Numbers corresponding to routes of migration are: ← Rostral migratory stream, ↑ dLGE aspect of the lateral cortical stream to basal lateral limbic system, → MGE to cerebral cortex, ↓ MGE to LGE, ⊗ MGE to amygdala ⊕ CGE to cerebral cortex Ø CGE to amygdala. Table of the source GABA progenitor pool, final destination in the mature brain and cell fate is shown in (C). Question marks indicate that the final destination or cell fate of a specific progenitor pool remains speculative. *Note, for simplicity, the LGE and MGE are not shown as being subdivided into smaller progenitor pools.
Figure 3. Precursor diversity in the neocortical wall
Recent in vitro and in vivo studies have determined that the neocortical ventricular zone (VZ) contains multiple types of precursors, including several different types of radial glial cells (RGCs) and short neural precursors (SNPs). While it has been established that all VZ precursors can either directly or indirectly give rise to neurons, neither the lineage potential of each cell type nor the lineal relationships between the multiple VZ cell types have yet been established.
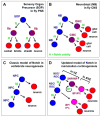
Figure 4. Notch function in drosophila and mammalian neurogenesis
(A) Cell fate specification in the drosophila peripheral nervous system (PNS). At each of the three cell divisions Notch signaling (N) regulates a binary fate choice. The generation of a glial precursor early in the lineage has been omitted for simplicity. (B) Cell fate specification in the drosophila central nervous system (CNS). A neuroblast (NB) is maintained by Notch signaling during self-renewal while generating a ganglion mother cell (GMC) divides again to generate mature cell types. The fate of cell generated by the GMC is also regulated by Notch. (C) Classic and oversimplified view of Notch function during vertebrate neurogenesis. Notch functions to maintain neural stem cell/progenitors (NPCs) and to inhibit neurogenesis. Reduced Notch signaling permits the generation of neurons. (D) An updated model of Notch function during neocortical development in mammals. Canonical CBF1-mediated Notch signaling (N) maintains the neural stem cell (NSC) pool in the ventricular zone (VZ) in the form of radial glial cells (RGCs) (Gaiano and Fishell 2002). NSC/RGCs may generate short neural precursors (SNPs) in the VZ (Gal et al. 2006), which have also been called intermediate neural progenitors (INPs) (Mizutani et al. 2007). SNP/INPs are maintained by Notch signaling, but with attenuated CBF1-Hes activity (N*) (Mizutani et al. 2007). SNP/INP daughters can move to the SVZ as basal progenitors (BPs, are also called intermediate progenitor cells or IPCs), or may generate neurons directly (not depicted). In addition, NSC/RGCs have been observed to give rise directly to BP/IPCs (Noctor et al. 2004).
Figure 5. Radial glial signaling pathways
These pathways putatitvely influence the morphology of radial glia, as well as the ability of radial glia to provide a scaffold for migrating neurons and generate new neurons. The classic reelin pathway involves VLDL and ApoER2 receptors and Dab1 as shown on the left in shades of pink; PI3K is also an integral part of reelin signaling. Neuregulin (NRG1) signaling progresses primarily through erbB receptors and pathways leading to PI3K activation (shades of green) or involvement of the Notch signaling pathway (indicated in shades of purple). Activation of Notch results in upregulation of BLBP production. In certain radial glial cells, blockade of PI3K can be compensated by NRG1 signaling through erbB receptors that coordinate with Notch.
Similar articles
- Notch3 signaling promotes radial glial/progenitor character in the mammalian telencephalon.
Dang L, Yoon K, Wang M, Gaiano N. Dang L, et al. Dev Neurosci. 2006;28(1-2):58-69. doi: 10.1159/000090753. Dev Neurosci. 2006. PMID: 16508304 - Dorsal-ventral patterning in the mammalian telencephalon.
Campbell K. Campbell K. Curr Opin Neurobiol. 2003 Feb;13(1):50-6. doi: 10.1016/s0959-4388(03)00009-6. Curr Opin Neurobiol. 2003. PMID: 12593982 Review. - Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains.
Imayoshi I, Sakamoto M, Yamaguchi M, Mori K, Kageyama R. Imayoshi I, et al. J Neurosci. 2010 Mar 3;30(9):3489-98. doi: 10.1523/JNEUROSCI.4987-09.2010. J Neurosci. 2010. PMID: 20203209 Free PMC article. - Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon.
Ganz J, Kaslin J, Hochmann S, Freudenreich D, Brand M. Ganz J, et al. Glia. 2010 Aug 15;58(11):1345-63. doi: 10.1002/glia.21012. Glia. 2010. PMID: 20607866 - Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain.
Corbin JG, Nery S, Fishell G. Corbin JG, et al. Nat Neurosci. 2001 Nov;4 Suppl:1177-82. doi: 10.1038/nn749. Nat Neurosci. 2001. PMID: 11687827 Review.
Cited by
- Serotonin homeostasis and serotonin receptors as actors of cortical construction: special attention to the 5-HT3A and 5-HT6 receptor subtypes.
Vitalis T, Ansorge MS, Dayer AG. Vitalis T, et al. Front Cell Neurosci. 2013 Jun 19;7:93. doi: 10.3389/fncel.2013.00093. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23801939 Free PMC article. - Apical Polarization of SVCT2 in Apical Radial Glial Cells and Progenitors During Brain Development.
Silva-Álvarez C, Salazar K, Cisternas P, Martínez F, Liour S, Jara N, Bertinat R, Nualart F. Silva-Álvarez C, et al. Mol Neurobiol. 2017 Sep;54(7):5449-5467. doi: 10.1007/s12035-016-0081-2. Epub 2016 Sep 5. Mol Neurobiol. 2017. PMID: 27596508 - Wired for behaviors: from development to function of innate limbic system circuitry.
Sokolowski K, Corbin JG. Sokolowski K, et al. Front Mol Neurosci. 2012 Apr 26;5:55. doi: 10.3389/fnmol.2012.00055. eCollection 2012. Front Mol Neurosci. 2012. PMID: 22557946 Free PMC article. - Multicolor lineage tracing using in vivo time-lapse imaging reveals coordinated death of clonally related cells in the developing vertebrate brain.
Brockway NL, Cook ZT, O'Gallagher MJ, Tobias ZJC, Gedi M, Carey KM, Unni VK, Pan YA, Metz MR, Weissman TA. Brockway NL, et al. Dev Biol. 2019 Sep 15;453(2):130-140. doi: 10.1016/j.ydbio.2019.05.006. Epub 2019 May 16. Dev Biol. 2019. PMID: 31102591 Free PMC article. - Shared effects of DISC1 disruption and elevated WNT signaling in human cerebral organoids.
Srikanth P, Lagomarsino VN, Muratore CR, Ryu SC, He A, Taylor WM, Zhou C, Arellano M, Young-Pearse TL. Srikanth P, et al. Transl Psychiatry. 2018 Apr 12;8(1):77. doi: 10.1038/s41398-018-0122-x. Transl Psychiatry. 2018. PMID: 29643329 Free PMC article.
References
- Aboitiz F, Montiel J. Co-option of signaling mechanisms from neural induction to telencephalic patterning. Rev Neurosci. 2007;18:311–342. - PubMed
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–897. - PubMed
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. - PubMed
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical