A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial - PubMed (original) (raw)
Clinical Trial
. 2008 Nov 25;26(50):6338-43.
doi: 10.1016/j.vaccine.2008.09.026. Epub 2008 Sep 26.
Mark K Louder, LaSonji A Holman, Ingelise J Gordon, Mary E Enama, Brenda D Larkin, Charla A Andrews, Leatrice Vogel, Richard A Koup, Mario Roederer, Robert T Bailer, Phillip L Gomez, Martha Nason, John R Mascola, Gary J Nabel, Barney S Graham; VRC 301 Study Team
Collaborators, Affiliations
- PMID: 18824060
- PMCID: PMC2612543
- DOI: 10.1016/j.vaccine.2008.09.026
Clinical Trial
A SARS DNA vaccine induces neutralizing antibody and cellular immune responses in healthy adults in a Phase I clinical trial
Julie E Martin et al. Vaccine. 2008.
Abstract
Background: The severe acute respiratory syndrome (SARS) virus is a member of the Coronaviridae (CoV) family that first appeared in the Guangdong Province of China in 2002 and was recognized as an emerging infectious disease in March 2003. Over 8000 cases and 900 deaths occurred during the epidemic. We report the safety and immunogenicity of a SARS DNA vaccine in a Phase I human study.
Methods: A single-plasmid DNA vaccine encoding the Spike (S) glycoprotein was evaluated in 10 healthy adults. Nine subjects completed the 3 dose vaccination schedule and were evaluated for vaccine safety and immune responses. Immune response was assessed by intracellular cytokine staining (ICS), ELISpot, ELISA, and neutralization assays.
Results: The vaccine was well tolerated. SARS-CoV-specific antibody was detected by ELISA in 8 of 10 subjects and neutralizing antibody was detected in all subjects who received 3 doses of vaccine. SARS-CoV-specific CD4+ T-cell responses were detected in all vaccinees, and CD8+ T-cell responses in approximately 20% of individuals.
Conclusions: The VRC SARS DNA vaccine was well tolerated and produced cellular immune responses and neutralizing antibody in healthy adults.
Figures
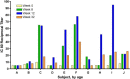
Fig. 1
Magnitude and frequency of neutralizing antibody response. Individual subjects are designated by letters A–J, sorted by ascending age on the _x_-axis. The IC80 (inhibitory concentration 80%) reciprocal titer is represented on the _y_-axis. The time course of the study is shown for each subject: week 0 (yellow bars), week 8 (green bars), week 12 (blue bars), and week 32 (orange bars). Vaccinations were administered at weeks 0, 4, and 8. Subject “G” received 2 of 3 vaccinations.
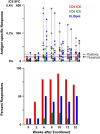
Fig. 2
Magnitude and frequency of CD4 and CD8 T-cell responses by ICS and ELISpot analysis at specific timepoints throughout the study. Magnitude of response is represented on the upper graph, percent positive CD4 (red bars) or CD8 cells (green bars) for ICS or spot-forming colonies (SFC) for ELISpot (blue bars). The horizontal black bars represent the mean. A sample was considered positive if it was above the thresholds indicated by the dashed lines. Separate positivity criteria for CD4 and CD8 ICS and ELISpot were developed and validated for overlapping peptide-based stimulations. The frequency of response is represented by percent responders on the lower graph. Weeks after enrollment is shown on the _x_-axis, applicable for upper and lower graphs. Vaccinations were administered at weeks 0, 4, and 8.
Similar articles
- Priming with SARS CoV S DNA and boosting with SARS CoV S epitopes specific for CD4+ and CD8+ T cells promote cellular immune responses.
Huang J, Cao Y, Du J, Bu X, Ma R, Wu C. Huang J, et al. Vaccine. 2007 Sep 28;25(39-40):6981-91. doi: 10.1016/j.vaccine.2007.06.047. Epub 2007 Jul 16. Vaccine. 2007. PMID: 17709158 Free PMC article. - Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice.
Hu H, Lu X, Tao L, Bai B, Zhang Z, Chen Y, Zheng F, Chen J, Chen Z, Wang H. Hu H, et al. Clin Vaccine Immunol. 2007 Jul;14(7):894-901. doi: 10.1128/CVI.00019-07. Epub 2007 May 9. Clin Vaccine Immunol. 2007. PMID: 17494640 Free PMC article. - Comparative evaluation of two severe acute respiratory syndrome (SARS) vaccine candidates in mice challenged with SARS coronavirus.
See RH, Zakhartchouk AN, Petric M, Lawrence DJ, Mok CPY, Hogan RJ, Rowe T, Zitzow LA, Karunakaran KP, Hitt MM, Graham FL, Prevec L, Mahony JB, Sharon C, Auperin TC, Rini JM, Tingle AJ, Scheifele DW, Skowronski DM, Patrick DM, Voss TG, Babiuk LA, Gauldie J, Roper RL, Brunham RC, Finlay BB. See RH, et al. J Gen Virol. 2006 Mar;87(Pt 3):641-650. doi: 10.1099/vir.0.81579-0. J Gen Virol. 2006. PMID: 16476986 - SARS Immunity and Vaccination.
Zhu M. Zhu M. Cell Mol Immunol. 2004 Jun;1(3):193-8. Cell Mol Immunol. 2004. PMID: 16219167 Review. - Vaccine design for severe acute respiratory syndrome coronavirus.
He Y, Jiang S. He Y, et al. Viral Immunol. 2005;18(2):327-32. doi: 10.1089/vim.2005.18.327. Viral Immunol. 2005. PMID: 16035944 Review.
Cited by
- Influenza virus h5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans.
Ledgerwood JE, Hu Z, Gordon IJ, Yamshchikov G, Enama ME, Plummer S, Bailer R, Pearce MB, Tumpey TM, Koup RA, Mascola JR, Nabel GJ, Graham BS; VRC 304 and VRC 305 Study Teams. Ledgerwood JE, et al. Clin Vaccine Immunol. 2012 Nov;19(11):1792-7. doi: 10.1128/CVI.05663-11. Epub 2012 Sep 5. Clin Vaccine Immunol. 2012. PMID: 22956656 Free PMC article. Clinical Trial. - A systematic review of SARS-CoV-2 vaccine candidates.
Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F. Dong Y, et al. Signal Transduct Target Ther. 2020 Oct 13;5(1):237. doi: 10.1038/s41392-020-00352-y. Signal Transduct Target Ther. 2020. PMID: 33051445 Free PMC article. - Lessons for COVID-19 Immunity from Other Coronavirus Infections.
Sariol A, Perlman S. Sariol A, et al. Immunity. 2020 Aug 18;53(2):248-263. doi: 10.1016/j.immuni.2020.07.005. Epub 2020 Jul 14. Immunity. 2020. PMID: 32717182 Free PMC article. Review. - Immunoinformatics-guided design of a multi-epitope vaccine based on the structural proteins of severe acute respiratory syndrome coronavirus 2.
Obaidullah AJ, Alanazi MM, Alsaif NA, Albassam H, Almehizia AA, Alqahtani AM, Mahmud S, Sami SA, Emran TB. Obaidullah AJ, et al. RSC Adv. 2021 May 19;11(29):18103-18121. doi: 10.1039/d1ra02885e. eCollection 2021 May 13. RSC Adv. 2021. PMID: 35480208 Free PMC article. - Immunotherapy of SARS based on combinations of neutralizing human monoclonal antibodies.
Du L, Jiang S. Du L, et al. Future Virol. 2010 Mar;5(2):141-144. doi: 10.2217/fvl.09.78. Epub 2010 Mar 1. Future Virol. 2010. PMID: 32201501 Free PMC article.
References
- Cumulative number of reported probable cases of SARS. 2003.
- Guan Y., Zheng B.J., He Y.Q., Liu X.L., Zhuang Z.X., Cheung C.L. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003;302(5643):276–278. - PubMed
- Holmes K.V. SARS-associated coronavirus. N Engl J Med. 2003;348(20):1948–1951. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous