High-throughput T7 LIC vector for introducing C-terminal poly-histidine tags with variable lengths without extra sequences - PubMed (original) (raw)
High-throughput T7 LIC vector for introducing C-terminal poly-histidine tags with variable lengths without extra sequences
Jonas Lee et al. Protein Expr Purif. 2009 Jan.
Abstract
Immobilized metal ion affinity chromatography (IMAC) has become one of the most popular protein purification methods for recombinant proteins with a hexa-histidine tag (His-tag) placed at the C- or N-terminus of proteins. Nevertheless, there are always difficult proteins that show weak binding to the metal chelating resin and thus low purity. These difficulties are often overcome by increasing the His-tag to 8 or 10 histidines. Despite their success, there are only few expression vectors available to easily clone and test different His-tag lengths. Therefore, we have modified Escherichia coli T7 expression vector pET21a to accommodate ligation-independent cloning (LIC) that will allow easy and efficient parallel cloning of target genes with different His-tag lengths using a single insert. Unlike most LIC vectors available commercially, our vectors will not translate unwanted extra sequences by engineering the N-terminal linker to anneal before the open reading frame, and the C-terminal linker to anneal as a His-tag.
Figures
Figure 1. Cloning Site of pJL and the LIC Reaction Scheme
pJL was derived from pL by changing the MCS by making three different variations to accommodate three different His-tag lengths. These vector variations are made to allow simultaneous cloning of proteins with three different His-tag lengths. The length of the His-tag can be further modified by inserting or removing histidine codons in the PCR primers of the C-terminus LIC linker.
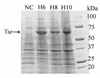
Figure 2. SDS-PAGE Gel of Expression Testing of pJL Clones in BL21(DE3) Star using ZYM autoinducing media
Expression of E. coli serine chemotaxis receptor (Tsr) in pJL vectors. 4–20% SDS-PAGE stained in Coomassie R-250 stain: Lane NC: Negative control, empty pJL-H6 vector; Lane H6: pJL-H6.Tsr; Lane H8: pJL-H8.Tsr; Lane H10: pJL-H10.Tsr. Tsr is a 59 kDa protein. All samples were grown in 2 mL ZYM-5052 media at 37°C, 200 RPM with overnight shaking (~16 hours). Estimated level of expressions are Tsr H6 – 200 mg/L or 25 mg/g of wet cells, Tsr H8 – 100 mg/L or 13 mg/g of wet cells, and Tsr H10 – 200 mg/L or 25 mg/g of wet cells.
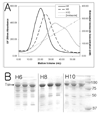
Figure 3. IMAC of pJL. Tsr with Different His-tag Lengths
A) UV 280 nm absorbance profile of different His-tag length Tsr elution. H6 – six histidine tag; H8 – eight histidine tag; H10 – ten histidine tag; [Imidazole] – estimated imidazole concentration in mM (corresponds to the right Y-axis scale). The UV absorbance of H10 was scaled up by 1.7X to match integrated peak areas of H6 and H8 for easier comparison of peak height and broadness. As the length of a His-tag increases, the eluting imidazole concentration and broadness of elution peak increases. UV absorbance maxima corresponds to 110 mM (H6), 133 mM (H8), and 180 mM (H10) imidazole. B) 12% SDS-PAGE gel of peak fractions. Each lane corresponds to 3 mL fractions collected at UV absorbance peaks which corresponds to the elution volume, H6 – Lane 1 :12 to 15 mL, Lane 2 : 15 to 18 mL, Lane 3 : 18 to 21 mL; H8 – Lane 1 : 21 to 24 mL, Lane 2 : 24 to 27 mL, Lane 3 : 27 to 30 mL; H10 – Lane 1 : 30 to 33 mL, Lane 2 : 33 to 36 mL, Lane 3 : 36 to 39 mL. Eight micrograms of a total protein, determined by Bradford assay, were loaded on each lane except the last lane (marker lane). The overall purity increases slightly as the length of a His-tag increases indicating a possibility that impurities interact with Tsr.
Similar articles
- A family of E. coli expression vectors for laboratory scale and high throughput soluble protein production.
Cabrita LD, Dai W, Bottomley SP. Cabrita LD, et al. BMC Biotechnol. 2006 Mar 1;6:12. doi: 10.1186/1472-6750-6-12. BMC Biotechnol. 2006. PMID: 16509985 Free PMC article. - Functional efficacy of human recombinant FGF-2s tagged with (His)6 and (His-Asn)6 at the N- and C-termini in human gingival fibroblast and periodontal ligament-derived cells.
Lee JH, Lee JE, Kang KJ, Jang YJ. Lee JH, et al. Protein Expr Purif. 2017 Jul;135:37-44. doi: 10.1016/j.pep.2017.05.001. Epub 2017 May 6. Protein Expr Purif. 2017. PMID: 28487257 - A new vector coupling ligation-independent cloning with sortase a fusion for efficient cloning and one-step purification of tag-free recombinant proteins.
Jia X, Crawford T, Zhang AH, Mobli M. Jia X, et al. Protein Expr Purif. 2019 Sep;161:1-7. doi: 10.1016/j.pep.2019.04.004. Epub 2019 Apr 22. Protein Expr Purif. 2019. PMID: 31022449 - Affinity Tags for Protein Purification.
Mishra V. Mishra V. Curr Protein Pept Sci. 2020;21(8):821-830. doi: 10.2174/1389203721666200606220109. Curr Protein Pept Sci. 2020. PMID: 32504500 Review. - Enhancing the solubility of recombinant proteins in Escherichia coli by using hexahistidine-tagged maltose-binding protein as a fusion partner.
Sun P, Tropea JE, Waugh DS. Sun P, et al. Methods Mol Biol. 2011;705:259-74. doi: 10.1007/978-1-61737-967-3_16. Methods Mol Biol. 2011. PMID: 21125392 Review.
Cited by
- Tagging the expressed protein with 6 histidines: rapid cloning of an amplicon with three options.
Singh MI, Jain V. Singh MI, et al. PLoS One. 2013 May 15;8(5):e63922. doi: 10.1371/journal.pone.0063922. Print 2013. PLoS One. 2013. PMID: 23691118 Free PMC article. - Development of a Novel Cell Surface Attachment System to Display Multi-Protein Complex Using the Cohesin-Dockerin Binding Pair.
Ko HJ, Song H, Choi IG. Ko HJ, et al. J Microbiol Biotechnol. 2021 Aug 28;31(8):1183-1189. doi: 10.4014/jmb.2105.05022. J Microbiol Biotechnol. 2021. PMID: 34226404 Free PMC article. - Functional cell surface display and controlled secretion of diverse Agarolytic enzymes by Escherichia coli with a novel ligation-independent cloning vector based on the autotransporter YfaL.
Ko HJ, Park E, Song J, Yang TH, Lee HJ, Kim KH, Choi IG. Ko HJ, et al. Appl Environ Microbiol. 2012 May;78(9):3051-8. doi: 10.1128/AEM.07004-11. Epub 2012 Feb 17. Appl Environ Microbiol. 2012. PMID: 22344647 Free PMC article. - Rapid and Robust PCR-Based All-Recombinant Cloning Methodology.
Dubey AA, Singh MI, Jain V. Dubey AA, et al. PLoS One. 2016 Mar 23;11(3):e0152106. doi: 10.1371/journal.pone.0152106. eCollection 2016. PLoS One. 2016. PMID: 27007922 Free PMC article. - Recombinant expressed vector pET32a (+) S constructed by ligation independent cloning.
Wang Y, Gong GH, Wei CX, Liang L, Zhang B. Wang Y, et al. Molecules. 2014 Oct 10;19(10):16179-89. doi: 10.3390/molecules191016179. Molecules. 2014. PMID: 25310147 Free PMC article.
References
- Porath J, Carlsson J, Olsson I, Belfrage G. Metal chelate affinity chromatography, a new approach to protein fractionation. Nature. 1975;258:598–599. - PubMed
- Porath J. Immobilized metal ion affinity chromatography. Protein Expr. Purif. 1992;3:263–281. - PubMed
- Gutierrez R, del Valle E, Galan M. Immobilized metal-ion affinity chromatography: status and trends. Separation. Purif. Rev. 2007;36:71–111.
- Gaberc-Porekar V, Menart V. Potential for using histidine tags in purification of proteins at large scale. Chem. Eng. Tech. 2005;28:1306–1314.
- Changa G. Twenty-five years of immobilized metal ion affinity chromatograph: past, present and future. Jour. Biochem. Biophys. Met. 2001;49:313–334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources