Induction- and conditioning-protocol dependent involvement of NR2B-containing NMDA receptors in synaptic potentiation and contextual fear memory in the hippocampal CA1 region of rats - PubMed (original) (raw)
Induction- and conditioning-protocol dependent involvement of NR2B-containing NMDA receptors in synaptic potentiation and contextual fear memory in the hippocampal CA1 region of rats
Xue-Han Zhang et al. Mol Brain. 2008.
Abstract
Long-term potentiation (LTP) in the hippocampal CA1 region requires the activation of N-methyl-D-aspartate receptors (NMDARs). Studies using genetic and pharmacological approaches have reported inconsistent results of the requirement of NR2B-containing NMDARs in LTP in the CA1 region. Pharmacological studies showed that NR2B-containing NMDARs are not required for LTP, while genetic studies reported that over-expression of NR2B-NMDARs enhances LTP and hippocampus-dependent memory. Here, we provide evidence showing that the functional role of NR2B-NMDARs in hippocampal LTP and memory depends on LTP-inducing and behavior-conditioning protocols. Inhibition of NR2B-NMDARs with the NR2B selective antagonist ifenprodil or Ro25-6981 suppressed LTP induced by spike-timing protocol, with no impact on LTP induced by pairing protocol or two-train high-frequency stimulation (HFS) protocol. Inhibition of NR2B-NMDARs did not affect the late phase LTP induced by four-train HFS. Ca²(+) imaging showed that there was difference in kinetics of intracellular Ca²(+) signals induced by spiking-timing and pairing protocols. Pre-training intra-CA1 infusion of ifenprodil or Ro25-6981 impaired the contextual fear memory induced by five CS-US pairings, with no effect on the memory induced by one CS-US pairing.
Figures
Figure 1
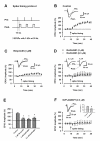
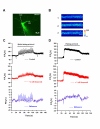
NR2B-NMDARs are required for LTP induced by spike-timing protocol in area CA1. A. Schematic diagram of the spike-timing protocol. B. Spike-timing protocol, as indicated by the arrow, induced a significant LTP in CA1 pyramidal neurons (n = 8). Sample traces of EPSC are the averages of 7 consecutive responses recorded during 5–10 min and 25–30 min, respectively. C. Bath application of ifenprodil partially blocked the LTP. n = 8 neurons D. Bath application of Ro25-6981 partially blocked the LTP. n = 7 neurons for 0.3 μM Ro25-6981; n = 8 neurons for 3 μM Ro25-6981. E. Histograms showing the effects of ifenprodil and Ro25-6981 on the LTP. *p < 0.05 vs. control. F. NR2A-NMDARs are required for LTP induced by spike-timing protocol in area CA1. Bath application of NVP-AAM077 blocked the LTP. n = 7 neurons. inset: Histogram showing the effect of NVP-AAM077 on the LTP. *p < 0.05 vs. control.
Figure 2
NR2B-NMDARs are not required for LTP induced by pairing protocol in area CA1. A. Schematic diagram of the pairing protocol. B. Pairing protocol, as indicated by the arrow, induced a significant LTP in CA1 pyramidal neurons (n = 6). Sample traces of EPSC are the averages of 7 consecutive responses recorded during 5–10 min and 25–30 min, respectively. C. Bath application of Ro25-6981 (0.5 μM) had no effect on the LTP (n = 5). D. Histogram showing the effect of Ro25-6981 on the LTP. p > 0.05 vs. control.
Figure 3
NR2B-NMDARs are not required for LTP induced by HFS protocol in area CA1. A. Schematic diagram of the high frequency stimulation (HFS; 2 train). B. LTP of field EPSP induced by the HFS in control (n = 10 slices) and in the presence of Ro25-6981 (n = 7 slices). Ro25-7981 had no effect on the LTP.
Figure 4
NR2B-NMDARs are not required for L-LTP induced by HFS protocol in area CA1. A. Schematic diagram of the high frequency stimulation (HFS; 4 trains). B. Late phase LTP of field EPSP induced by the HFS in control (n = 7 slices) and in the presence of Ro25-6981 (n = 5 slices). Ro25-7981 had no effect on the L-LTP.
Figure 5
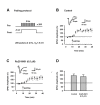
NR2B-NMDAR mediated Ca2+ influx under spiking-timing and pairing protocols. A. A representative image showing the CA1 pyramidal neuron filled with OGB-1. ROI, region of interests. B. Raw sample fluorescence images from the ROI before (1), during (2) and after (3) induction of LTP with the spike-timing protocol. Scale bar, 1.0 μm. C. Elevation of Ca2+ signal in the ROI during induction of LTP with the spike-timing protocol (upper). Treatment with ifenprodil (3 μM) reduced the Ca2+ signal (middle). The difference of [Ca2+] signals in control and in the presence of ifenprodil (lower) shows the NR2B-NMDAR mediated Ca2+ influx. D. Elevation of Ca2+ signal in the ROI during induction of LTP with the pairing protocol (upper). Treatment with ifenprodil (3 μM) reduced the Ca2+ signal (middle). The difference of [Ca2+] signals in control and in the presence of ifenprodil (lower) shows the NR2B-NMDAR mediated Ca2+ influx.
Figure 6
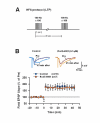
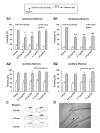
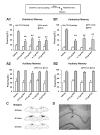
NR2B-NMDARs are required for the acquisition of contextual fear memory induced by the five but not one CS-US pairing conditioning. A. Pre-training intra-CA1 inhibition of NR2B-NMDARs had no impact on, while inhibition of NR2A-NMDARs impaired 48-h contextual fear memory induced by the single CS-US pairing protocol (A1). The acquisition of auditory fear memory was intact (A2). The result with Ro25-6981 was reported in the previous study (Zhao et al., 2005). *p < 0.05 vs. vehicle B. Pre-training intra-CA1 inhibition of NR2B- or NR2A-NMDARs impaired 48-h contextual fear memory induced by the five CS-US pairing protocol (B1). The acquisition of auditory fear memory was intact (B2). **p < 0.01 vs. vehicle. C. Reconstruction of the infusion sites in the CA1 region. Filled squares: vehicle; Open circles: 0.012 μg NVP-AAM077; Filled circles: 0.12 μg NVP-AAM077; Open triangles: ifenprodil; Grey squares: Ro25-6981. D. A representative coronal section showing an infusion site of ifenprodil in the CA1 region.
Figure 7
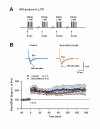
NR2B-NMDARs are required for the retrieval of contextual fear memory induced by both five and one CS-US pairing conditioning. A. Pre-retrieval intra-CA1 inhibition of NR2B- or NR2A-NMDARs impaired the expression of 48-h contextual fear memory induced by the single CS-US pairing protocol (A1). The expression of 48-h auditory fear memory was intact (A2). **p < 0.01 vs. vehicle. B. Pre-retrieval intra-CA1 inhibition of NR2B- or NR2A-NMDARs impaired the expression of 48-h contextual fear memory induced by the five CS-US pairing protocol (B1). The expression of 48-h auditory fear memory was intact (B2). **p < 0.01 vs. vehicle. C. Reconstruction of the infusion sites in the CA1 region. Filled squares: vehicle; Filled circles: NVP-AAM077; Open triangles: ifenprodil; Open circles: Ro25-6981. D. A representative coronal section showing an infusion site of ifenprodil in the CA1 region.
Similar articles
- Hippocampal NR2B-containing NMDA receptors enhance long-term potentiation in rats with chronic visceral pain.
Chen Y, Chen AQ, Luo XQ, Guo LX, Tang Y, Bao CJ, Lin L, Lin C. Chen Y, et al. Brain Res. 2014 Jun 27;1570:43-53. doi: 10.1016/j.brainres.2014.05.001. Epub 2014 May 10. Brain Res. 2014. PMID: 24824341 - Late phase of long-term potentiation induced by co-application of N-methyl-d-aspartic acid and the antagonist of NR2B-containing N-methyl-d-aspartic acid receptors in rat hippocampus.
Oh-Nishi A, Saji M, Satoh SZ, Ogata M, Suzuki N. Oh-Nishi A, et al. Neuroscience. 2009 Mar 3;159(1):127-35. doi: 10.1016/j.neuroscience.2008.10.037. Epub 2008 Oct 30. Neuroscience. 2009. PMID: 19010396 - Roles of NMDA NR2B subtype receptor in prefrontal long-term potentiation and contextual fear memory.
Zhao MG, Toyoda H, Lee YS, Wu LJ, Ko SW, Zhang XH, Jia Y, Shum F, Xu H, Li BM, Kaang BK, Zhuo M. Zhao MG, et al. Neuron. 2005 Sep 15;47(6):859-72. doi: 10.1016/j.neuron.2005.08.014. Neuron. 2005. PMID: 16157280 - Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.
MacDonald JF, Jackson MF, Beazely MA. MacDonald JF, et al. Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review. - Long-term potentiation and the role of N-methyl-D-aspartate receptors.
Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL. Volianskis A, et al. Brain Res. 2015 Sep 24;1621:5-16. doi: 10.1016/j.brainres.2015.01.016. Epub 2015 Jan 22. Brain Res. 2015. PMID: 25619552 Free PMC article. Review.
Cited by
- NMDA Receptor-Dependent Synaptic Depression in Potentiated Synapses of the Anterior Cingulate Cortex of adult Mice.
Xue M, Zhou SB, Liu RH, Chen QY, Zhuo M, Li XH. Xue M, et al. Mol Pain. 2021 Jan-Dec;17:17448069211018045. doi: 10.1177/17448069211018045. Mol Pain. 2021. PMID: 34024172 Free PMC article. - Memory enhancement by targeting Cdk5 regulation of NR2B.
Plattner F, Hernández A, Kistler TM, Pozo K, Zhong P, Yuen EY, Tan C, Hawasli AH, Cooke SF, Nishi A, Guo A, Wiederhold T, Yan Z, Bibb JA. Plattner F, et al. Neuron. 2014 Mar 5;81(5):1070-1083. doi: 10.1016/j.neuron.2014.01.022. Neuron. 2014. PMID: 24607229 Free PMC article. - Rapid synaptic potentiation within the anterior cingulate cortex mediates trace fear learning.
Descalzi G, Li XY, Chen T, Mercaldo V, Koga K, Zhuo M. Descalzi G, et al. Mol Brain. 2012 Feb 3;5:6. doi: 10.1186/1756-6606-5-6. Mol Brain. 2012. PMID: 22304729 Free PMC article. - Differences in GluN2B-Containing NMDA Receptors Result in Distinct Long-Term Plasticity at Ipsilateral versus Contralateral Cortico-Striatal Synapses.
Li W, Pozzo-Miller L. Li W, et al. eNeuro. 2019 Nov 27;6(6):ENEURO.0118-19.2019. doi: 10.1523/ENEURO.0118-19.2019. Print 2019 Nov/Dec. eNeuro. 2019. PMID: 31744842 Free PMC article. - Hippocampal NMDA receptor subunits differentially regulate fear memory formation and neuronal signal propagation.
Gao C, Gill MB, Tronson NC, Guedea AL, Guzmán YF, Huh KH, Corcoran KA, Swanson GT, Radulovic J. Gao C, et al. Hippocampus. 2010 Sep;20(9):1072-82. doi: 10.1002/hipo.20705. Hippocampus. 2010. PMID: 19806658 Free PMC article.
References
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous