Septins localize to microtubules during nutritional limitation in Saccharomyces cerevisiae - PubMed (original) (raw)
Septins localize to microtubules during nutritional limitation in Saccharomyces cerevisiae
M Evangelina Pablo-Hernando et al. BMC Cell Biol. 2008.
Abstract
Background: In Saccharomyces cerevisiae, nutrient limitation stimulates diploid cells to undergo DNA replication and meiosis, followed by the formation of four haploid spores. Septins are a family of proteins that assemble a ring structure at the mother-daughter neck during vegetative growth, where they control cytokinesis. In sporulating cells, the septin ring disassembles and septins relocalize to the prospore membrane.
Results: Here, we demonstrate that nutrient limitation triggers a change in the localization of at least two vegetative septins (Cdc10 and Cdc11) from the bud neck to the microtubules. The association of Cdc10 and Cdc11 with microtubules persists into meiosis, and they are found associated with the meiotic spindle until the end of meiosis II. In addition, the meiosis-specific septin Spr28 displays similar behavior, suggesting that this is a common feature of septins. Septin association to microtubules is a consequence of the nutrient limitation signal, since it is also observed when haploid cells are incubated in sporulation medium and when haploid or diploid cells are grown in medium containing non-fermentable carbon sources. Moreover, during meiosis II, when the nascent prospore membrane is formed, septins moved from the microtubules to this membrane. Proper organization of the septins on the membrane requires the sporulation-specific septins Spr3 and Spr28.
Conclusion: Nutrient limitation in S. cerevisiae triggers the sporulation process, but it also induces the disassembly of the septin bud neck ring and relocalization of the septin subunits to the nucleus. Septins remain associated with microtubules during the meiotic divisions and later, during spore morphogenesis, they are detected associated to the nascent prospore membranes surrounding each nuclear lobe. Septin association to microtubules also occurs during growth in non-fermentable carbon sources.
Figures
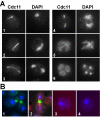
Figure 1
Cdc11 localization during meiosis. (A) Wild-type cells (AN120) incubated in sporulation medium were stained with anti-Cdc11 antibody and DAPI. Cells in different phases of meiosis are shown: (1), prophase; (2–3), anaphase I; (4), metaphase II; (5), anaphase II; (6), post-meiotic cell. (B) Exponentially growing wild-type cells were stained with anti-Cdc11 antibody (cell 1) or anti-Cdc11 and anti-tubulin antibodies (cell 2). Sporulating wild-type cells stained with anti-GFP and secondary anti-mouse Alexa 594 antibodies (cell 3) or the secondary antibodies anti-rabbit Alexa 488 and anti-mouse Alexa 633 (cell 4).
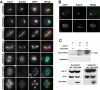
Figure 2
Cdc11 localizes to tubulin cytoskeleton during meiosis before its association with the prospore membrane. (A). Sporulating wild-type cells were stained with anti-Cdc11 and anti-tubulin antibodies and DAPI. Cells in different phases of meiosis are shown: (1), prophase; (2), metaphase I; (3) anaphase I; (4), metaphase II; (5), anaphase II; (6–7), post-meiotic cells. (B) Confocal microscopy of wild-type cells stained with anti-Cdc11 and anti-tubulin antibodies. The merged image shows Cdc11 (green) and tubulin (magenta) and white indicates areas where the two proteins colocalize. Cells in prophase (1) or anaphase II (2) are shown. (C) Cdc11 and Tub1 co-immunoprecipitate during sporulation. Protein extracts prepared from sporulating cells carrying TUB1-GFP (sporulation) or grown to mid-logarithmic phase (vegetative) were incubated in the presence (lane 3) or absence (lane 1) of anti-Cdc11 antibody. Extracts from the wild-type strain grown in the same conditions were used as controls (lane 2). Proteins were probed with anti-GFP antibody. The lower panels show a Western blot of the extracts used for the immunoprecipitations.
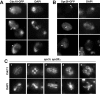
Figure 3
Cdc10 and Spr28 also localize with microtubules. (A) Wild-type cells carrying CDC10-GFP were stained with anti-GFP antibodies and DAPI. (B) Wild-type transformants carrying SPR28-GFP were stained with anti-GFP antibodies and DAPI. Arrowheads indicate Cdc10 (A) or Spr28 (B) localization to the microtubules. (C) Cdc11 localization in _spr3_Δ _spr28_Δ mutant. Sporulating cells (NY535) were stained with anti-Cdc11 antibody and DAPI. Cells in different phases of meiosis are shown: (1) anaphase I; (2), metaphase II; (3–4), anaphase II; (5), post-meiotic cells.
Figure 4
Localization of septins in the prospore membrane of sporulating cells. AN120 (wild-type), NY528 (_spr3_Δ) and NY703 (_spr28_Δ) strains were transformed with plasmids containing SPR3-GFP, SPR28-GFP, CDC3-GFP, CDC10-GFP or CDC11-GFP. Cells were sporulated, stained with DAPI and analyzed by fluorescence microscopy.
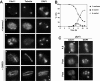
Figure 5
Cdc11 localization to microtubule does not require Gip1. (A) Sporulating wild-type cells were transferred to sporulation medium containing benomyl (120 μg/ml) (cells 1–3) or DMSO (cells 4–5) for 15 min and stained with anti-Cdc11 antibody and DAPI. Cells in different phases of meiosis are shown: (1–2), anaphase I; (3), anaphase II; (4), anaphase I; (5), anaphase II. (B) Progression through meiosis of cells in A. (C) _gip1_Δ mutants (NY501) were stained with anti-Cdc11 antibody and DAPI. Arrowheads indicate Cdc11 localized around the nascent prospore membranes. A II, anaphase II; post, post-meiotic cells.
Figure 6
Temporal pattern of Cdc11 localization to microtubules. (A) AN120 diploid cells (left panel) or AN117-16D haploid cells (right panel) were grown in YPAcetate medium before transferring to sporulation medium. Aliquots were then taken at 0, 1 and 2 h and stained with anti-Cdc11 antibody. The graph shows the percentage of cells with different Cdc11 stainings: bud neck (black circles), microtubules (black squares) or not localized (white squares). At least 200 cells were counted. (B) Wild-type cells (AN120) carrying _GFP-SPO20_51–91 (plasmid pG20) were sporulated and stained with anti-Cdc11 and DAPI. Images of Spo20-GFP, Cdc11 and DAPI are shown. The merged image shows Spo20 (green) and Cdc11 (magenta). Colocalization is indicated in white. Metaphase II (1–2), anaphase II (3), or post-meiotic (4) cells are shown.
Figure 7
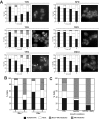
Non-fermentable carbon sources induce Cdc11 localization to microtubules. (A) AN117-16D haploid cells were inoculated in media containing glucose (YPD), acetate (YPA) or glycerol (YPG) as carbon sources or in media with poor nitrogen sources (SPD or MM-I). For MM-II medium, AN999 (MATα, lys1) was used. Cells were stained with anti-Cdc11 antibodies. The percentage of cells with bud neck (white), bud neck and microtubules (squares), microtubules (grey) or diffuse cytoplasmic Cdc11 staining (black) is shown in the graphs. Representative cells are also shown. (B) Cdc11 distribution in AN117-16D haploid cells or the _rho_- derivative grown in YPA. (C) AN117-16D haploid cells were grown in YPA for 16h and then transferred to AcK 2% or AcK 2% containing 15 mM sodium azide. The percentage of cells with different Cdc11 localizations 4 h after the inoculation is shown in the graph.
Similar articles
- Identification of a developmentally regulated septin and involvement of the septins in spore formation in Saccharomyces cerevisiae.
Fares H, Goetsch L, Pringle JR. Fares H, et al. J Cell Biol. 1996 Feb;132(3):399-411. doi: 10.1083/jcb.132.3.399. J Cell Biol. 1996. PMID: 8636217 Free PMC article. - SPR28, a sixth member of the septin gene family in Saccharomyces cerevisiae that is expressed specifically in sporulating cells.
De Virgilio C, DeMarini DJ, Pringle JR. De Virgilio C, et al. Microbiology (Reading). 1996 Oct;142 ( Pt 10):2897-905. doi: 10.1099/13500872-142-10-2897. Microbiology (Reading). 1996. PMID: 8885406 - Roles of septins in prospore membrane morphogenesis and spore wall assembly in Saccharomyces cerevisiae.
Heasley LR, McMurray MA. Heasley LR, et al. Mol Biol Cell. 2016 Feb 1;27(3):442-50. doi: 10.1091/mbc.E15-10-0721. Epub 2015 Dec 17. Mol Biol Cell. 2016. PMID: 26680739 Free PMC article. - Reuse, replace, recycle. Specificity in subunit inheritance and assembly of higher-order septin structures during mitotic and meiotic division in budding yeast.
McMurray MA, Thorner J. McMurray MA, et al. Cell Cycle. 2009 Jan 15;8(2):195-203. doi: 10.4161/cc.8.2.7381. Cell Cycle. 2009. PMID: 19164941 Free PMC article. Review. - Septin mutations and phenotypes in S. cerevisiae.
Mela A, Momany M. Mela A, et al. Cytoskeleton (Hoboken). 2019 Jan;76(1):33-44. doi: 10.1002/cm.21492. Epub 2018 Nov 18. Cytoskeleton (Hoboken). 2019. PMID: 30171672 Review.
Cited by
- Cellular functions of actin- and microtubule-associated septins.
Spiliotis ET, Nakos K. Spiliotis ET, et al. Curr Biol. 2021 May 24;31(10):R651-R666. doi: 10.1016/j.cub.2021.03.064. Curr Biol. 2021. PMID: 34033796 Free PMC article. Review. - Assembly, molecular organization, and membrane-binding properties of development-specific septins.
Garcia G 3rd, Finnigan GC, Heasley LR, Sterling SM, Aggarwal A, Pearson CG, Nogales E, McMurray MA, Thorner J. Garcia G 3rd, et al. J Cell Biol. 2016 Feb 29;212(5):515-29. doi: 10.1083/jcb.201511029. J Cell Biol. 2016. PMID: 26929450 Free PMC article. - Differential localization patterns of septins during growth of the human fungal pathogen Aspergillus fumigatus reveal novel functions.
Juvvadi PR, Fortwendel JR, Rogg LE, Steinbach WJ. Juvvadi PR, et al. Biochem Biophys Res Commun. 2011 Feb 11;405(2):238-43. doi: 10.1016/j.bbrc.2011.01.017. Epub 2011 Jan 8. Biochem Biophys Res Commun. 2011. PMID: 21219860 Free PMC article. - SPO71 mediates prospore membrane size and maturation in Saccharomyces cerevisiae.
Parodi EM, Baker CS, Tetzlaff C, Villahermosa S, Huang LS. Parodi EM, et al. Eukaryot Cell. 2012 Oct;11(10):1191-200. doi: 10.1128/EC.00076-12. Epub 2012 May 18. Eukaryot Cell. 2012. PMID: 22611022 Free PMC article. - Septins: molecular partitioning and the generation of cellular asymmetry.
McMurray MA, Thorner J. McMurray MA, et al. Cell Div. 2009 Aug 26;4:18. doi: 10.1186/1747-1028-4-18. Cell Div. 2009. PMID: 19709431 Free PMC article.
References
- Esposito RE, Klapholtz S. Meiosis and ascospore development. In: Strathern JN, Jones EW, Broach JR, editor. The Molecular and Cellular Biology of the Yeast Saccharomyces cerevisiae. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory; 1981. pp. 211–287.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases