Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis - PubMed (original) (raw)
Functional alterations of the ubiquitin-proteasome system in motor neurons of a mouse model of familial amyotrophic lateral sclerosis
Cristina Cheroni et al. Hum Mol Genet. 2009.
Abstract
In familial and sporadic amyotrophic lateral sclerosis (ALS) and in rodent models of the disease, alterations in the ubiquitin-proteasome system (UPS) may be responsible for the accumulation of potentially harmful ubiquitinated proteins, leading to motor neuron death. In the spinal cord of transgenic mice expressing the familial ALS superoxide dismutase 1 (SOD1) gene mutation G93A (SOD1G93A), we found a decrease in constitutive proteasome subunits during disease progression, as assessed by real-time PCR and immunohistochemistry. In parallel, an increased immunoproteasome expression was observed, which correlated with a local inflammatory response due to glial activation. These findings support the existence of proteasome modifications in ALS vulnerable tissues. To functionally investigate the UPS in ALS motor neurons in vivo, we crossed SOD1G93A mice with transgenic mice that express a fluorescently tagged reporter substrate of the UPS. In double-transgenic Ub(G76V)-GFP /SOD1G93A mice an increase in Ub(G76V)-GFP reporter, indicative of UPS impairment, was detectable in a few spinal motor neurons and not in reactive astrocytes or microglia, at symptomatic stage but not before symptoms onset. The levels of reporter transcript were unaltered, suggesting that the accumulation of Ub(G76V)-GFP was due to deficient reporter degradation. In some motor neurons the increase of Ub(G76V)-GFP was accompanied by the accumulation of ubiquitin and phosphorylated neurofilaments, both markers of ALS pathology. These data suggest that UPS impairment occurs in motor neurons of mutant SOD1-linked ALS mice and may play a role in the disease progression.
Figures
Figure 1.
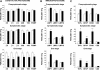
Real-time PCR in the lumbar spinal cord of pre-symptomatic, symptomatic and end-stage SOD1G93A mice (black column) and NTg littermates (white column) for the following mRNAs: (A) β5, β1, β2, α5 subunit of 20S, S1 subunit of 19S and POMP; (B) LMP7, LMP2 and LMP10; (C) TNFα, GFAP, CD68 and CD8 transcripts. (A) Significant decrease of 19S at pre-symptomatic stage and of α5 subunit at the symptomatic stage as compared with NTg littermates. A remarkable decrease is found in the mRNA levels of almost all subunits in SOD1G93A mice at the end-stage in respect to NTg littermates. (B) Progressive increase of LMP7 mRNA from the pre-symptomatic to the end-stage. A small but significant increase of LMP10 is found only at the symptomatic phase, while LMP2 never changes. (C) High significant increase of TNFα, CD68 and GFAP starting from pre-symptomatic to the final stage. All transcripts were normalized versus β-actin and the ratios expressed as percentage of the values from NTg littermates. Each histogram shows the mean ± SEM of at least four mice. Data were analyzed by Student’s _t_-test. (*P < 0.05; **P < 0.01; ***P < 0.001 compared with NTg).
Figure 2.
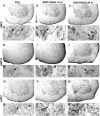
Immunoperoxidase detection of the proteasome inducible subunits LMP2, LMP10, LMP7 in lumbar spinal cord sections of NTg and SOD1G93A mice at pre-symptomatic (12 weeks) and late symptomatic (20 weeks) age. For each subunit the upper panels show low magnification images of the ventral horn, whereas the lower panels show details of motor neurons (arrows in D, E, G, K, L, N, R, S and U) and glial cells (F, M, T and V); asterisks = vacuoles in N. All the subunits are more intensely expressed in SOD1G93A mice than in NTg mice. Scale bar 10 µm in A–C, H–J, O–Q; 4 µm in D–G, K–N, R–U.
Figure 3.
Confocal images of proteasome inducible subunits LMP2, LMP10, LMP7 (green) in lumbar spinal cord sections of NTg (A, H, and N), SOD1wt mice (B, I, and O) and SOD1G93A mice (C–G′; J–M′, P–T′) at different ages. In NTg (A, H and N) and SOD1wt (B, I and O) mice motor neurons (asterisks) have very low immunoreactivity (green) for the three subunits. Immunoproteasome labeling (green) is intense in motor neurons (asterisks) of pre-symptomatic (C, J and P) and symptomatic (D, E, K and Q) SOD1G93A mice; some of the motor neurons are vacuolated (E and J) and accumulate human SOD1 (red in E, J′ and K′); R shows vacuoles (v) in the neuropil rimmed by LMP7 (green) and human SOD1 (red) labeling. In SOD1G93A mice the immunoreativity for the three subunits (green) is also in astrocytes identified by GFAP (red, arrowheads in F, F′, L, L′, S and S′) and in microglial cells identified by CD11β (red in G, G′, M, M′, T and T′). Scale bar 4 µm in A–D, H–K, N–Q; 3.8 µm in E; 3 µm in F, L, M and S; 2.8 µm in G; 2.5 µm in T.
Figure 4.
Colocalization of GFP and SMI-32 immunoreactivity in spinal neurons cultures from GFP2 mouse embryos after proteasome inhibition. Cultures are treated with vehicle (A–C) or with the proteasome inhibitor MG132 1.5 μ
m
(D–F) to induce an increase of GFP levels. An intense GFP immunostaining is observed in motor neurons characterized by an intense SMI-32 labeling after proteasome inhibition. Scale bar 50 µm, insets 20 µm.
Figure 5.
(A) Representative immunoblot for UbG76V-GFP in the lumbar spinal cord of GFP1 and GFP1/SOD1G93A mice at the symptomatic stage. (B) Quantitative analysis of the immunoblot. The optical density was measured for each autoradiographic band and values for GFP1/SOD1G93A were compared with GFP1 after correction for total protein. Each histogram shows the mean ± SEM of at least four mice. Data were analyzed by Student’s _t_-test. P = 0.058 (C) Real-time PCR for UbG76V-GFP transcript in the lumbar spinal cord of GFP1/SOD1G93A mice and GFP1 littermates at the symptomatic stage of disease progression. Levels of GFP transcript were normalized to β-actin and the ratios from double-transgenic mice were expressed as percentage of the ratio value of the GFP1 mice. Each histogram shows the mean ± SEM of at least five mice. Data were analyzed by Student’s _t_-test.
Figure 6.
Immunohistochemistry of GFP in the dorsal and ventral horns of the lumbar spinal cord of symptomatic SOD1G93A (A and B), and GFP2/SOD1G93A (E and F) mice and in age matched GFP2 mice (C and D). While in sections from SOD1G93A and GFP2 mice the reporter protein remains undetectable (A–D), in the ventral horn of symptomatic GFP2/SOD1G93A mice a clear accumulation of the reporter protein occurs in motor neuronal-like cells; some of them show morphological alterations such as vacuolization (framed neurons in F, G and H). Scale bar 100 µm (A–F); 20 µm (G and H).
Figure 7.
Representative microphotographs of GFP immunostaining in sagital sections from hindbrain and cerebellum of GFP2 (A and D) and GFP2/SOD1G93A mice at the end-stage (B, C, E and F). GFP signal is barely detectable in the hindbrain of GFP2 mice while it is remarkably intense in the same region of GFP2/SOD1G93A mice. Intense GFP-positive neuronal-like cells are found in nuclei trigemini (C) and facialis (F) of double-transgenic mice. Low GFP immunoreactivity is detectable in the cerebellum and no differences are found between the two mouse lines. Scale bar 500 µm (A, B, D and E); 20 µm (C and F).
Figure 8.
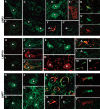
(A) Representative laser scanning confocal microphotographs of colocalization of GFP and ChAT immunostaining in the ventral horn of the lumbar spinal cord from GFP2 (A–C) or GFP2/SOD1G93A mice at the symptomatic stage (D–F). As shown in merge (C and F), intense GFP immunostaining is observed in some ChAT motor neurons (inset in F) of GFP2/SOD1G93A mice. Scale bar 100 µm (inset 1500×). (B) Representative laser scanning confocal microphotographs of GFP and GFAP or CD11β immunostaining in the ventral horn of the lumbar spinal cord of GFP2 (G and J), SOD1G93A (H and K) and symptomatic GFP2/SOD1G93A (I and L) mice. No colocalization is found with GFAP and CD11β. Scale bar 50 µm.
Figure 9.
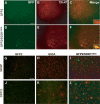
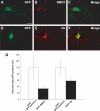
Representative laser scanning confocal microphotographs of GFP (A and D) and SMI-31 (B) or ubiquitin (E) immunostaining on ventral horn of the lumbar spinal cord of symptomatic GFP2/SOD1G93A mice. Colocalization is found between GFP and ubiquitin (C) or SMI-31 (F) labeling. Scale bar 20 µm. (G) Quantitative analysis of SMI-31 or ubiquitin-labeled neurons within GFP-positive population in the ventral horns of symptomatic GFP2/SOD1G93A mice. White histogram represents total GFP-positive cells, expressed in percentage (white bars, mean ± SEM of five mice), while black histogram represents the fraction that is also SMI-31 or ubiquitin-positive (black bars, mean ± SEM of five mice).
Figure 10.
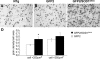
Representative autoradiographs of lumbar spinal cord sections from NTg (A), GFP2 (B) and symptomatic GFP2/SOD1G93A mice (C) hybridized with 35S-labelled anti-sense GFP-RNA probe. An intense silver grain density is observed in the cells of GFP2/SOD1G93A sections (B and C). Scale bar 100 µm. (D) Quantification of the grain density in cells from the ventral horn lumbar spinal cord of GFP2 and GFP2/SOD1G93A mice. Each histogram represents the mean ± SEM of five mice. Data were analyzed by Student’s _t_-test (*P < 0.05 compared with GFP). In symptomatic GFP2/SOD1G93A a significant increase of UbG76V-GFP mRNA is detected in small cells (<250 µm2), but not in large cells (>250 µm2) compared with GFP2 mice.
Similar articles
- Accumulation of human SOD1 and ubiquitinated deposits in the spinal cord of SOD1G93A mice during motor neuron disease progression correlates with a decrease of proteasome.
Cheroni C, Peviani M, Cascio P, Debiasi S, Monti C, Bendotti C. Cheroni C, et al. Neurobiol Dis. 2005 Apr;18(3):509-22. doi: 10.1016/j.nbd.2004.12.007. Neurobiol Dis. 2005. PMID: 15755678 - Bee venom effects on ubiquitin proteasome system in hSOD1(G85R)-expressing NSC34 motor neuron cells.
Kim SH, Jung SY, Lee KW, Lee SH, Cai M, Choi SM, Yang EJ. Kim SH, et al. BMC Complement Altern Med. 2013 Jul 18;13:179. doi: 10.1186/1472-6882-13-179. BMC Complement Altern Med. 2013. PMID: 23866691 Free PMC article. - Microglia and motor neurons during disease progression in the SOD1G93A mouse model of amyotrophic lateral sclerosis: changes in arginase1 and inducible nitric oxide synthase.
Lewis KE, Rasmussen AL, Bennett W, King A, West AK, Chung RS, Chuah MI. Lewis KE, et al. J Neuroinflammation. 2014 Mar 23;11:55. doi: 10.1186/1742-2094-11-55. J Neuroinflammation. 2014. PMID: 24655927 Free PMC article. - Dysfunction of constitutive and inducible ubiquitin-proteasome system in amyotrophic lateral sclerosis: implication for protein aggregation and immune response.
Bendotti C, Marino M, Cheroni C, Fontana E, Crippa V, Poletti A, De Biasi S. Bendotti C, et al. Prog Neurobiol. 2012 May;97(2):101-26. doi: 10.1016/j.pneurobio.2011.10.001. Epub 2011 Oct 20. Prog Neurobiol. 2012. PMID: 22033150 Review. - Rodent Models of Amyotrophic Lateral Sclerosis.
Philips T, Rothstein JD. Philips T, et al. Curr Protoc Pharmacol. 2015 Jun 1;69:5.67.1-5.67.21. doi: 10.1002/0471141755.ph0567s69. Curr Protoc Pharmacol. 2015. PMID: 26344214 Free PMC article. Review.
Cited by
- The Ubiquitin Receptor ADRM1 Modulates HAP40-Induced Proteasome Activity.
Huang ZN, Her LS. Huang ZN, et al. Mol Neurobiol. 2017 Nov;54(9):7382-7400. doi: 10.1007/s12035-016-0247-y. Epub 2016 Nov 5. Mol Neurobiol. 2017. PMID: 27815841 - Muscle Involvement in Amyotrophic Lateral Sclerosis: Understanding the Pathogenesis and Advancing Therapeutics.
Duranti E, Villa C. Duranti E, et al. Biomolecules. 2023 Oct 26;13(11):1582. doi: 10.3390/biom13111582. Biomolecules. 2023. PMID: 38002264 Free PMC article. Review. - Dopaminergic neurons show increased low-molecular-mass protein 7 activity induced by 6-hydroxydopamine in vitro and in vivo.
Mo MS, Li GH, Sun CC, Huang SX, Wei L, Zhang LM, Zhou MM, Wu ZH, Guo WY, Yang XL, Chen CJ, Qu SG, He JX, Xu PY. Mo MS, et al. Transl Neurodegener. 2018 Aug 17;7:19. doi: 10.1186/s40035-018-0125-9. eCollection 2018. Transl Neurodegener. 2018. PMID: 30128145 Free PMC article. - Sequestosome 1/p62 links familial ALS mutant SOD1 to LC3 via an ubiquitin-independent mechanism.
Gal J, Ström AL, Kwinter DM, Kilty R, Zhang J, Shi P, Fu W, Wooten MW, Zhu H. Gal J, et al. J Neurochem. 2009 Nov;111(4):1062-73. doi: 10.1111/j.1471-4159.2009.06388.x. Epub 2009 Sep 18. J Neurochem. 2009. PMID: 19765191 Free PMC article. - Protein quality control during erythropoiesis and hemoglobin synthesis.
Khandros E, Weiss MJ. Khandros E, et al. Hematol Oncol Clin North Am. 2010 Dec;24(6):1071-88. doi: 10.1016/j.hoc.2010.08.013. Hematol Oncol Clin North Am. 2010. PMID: 21075281 Free PMC article. Review.
References
- Rowland L.P., Shneider N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 2001;344:1688–1700. - PubMed
- Pasinelli P., Brown R.H. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nat. Rev. Neurosci. 2006;7:710–723. - PubMed
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
- Gurney M.E., Pu H., Chiu A.Y., Dal Canto M.C., Polchow C.Y., Alexander D.D., Caliendo J., Hentati A., Kwon Y.W., Deng H.X., et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. - PubMed
- Bendotti C., Carri M.T. Lessons from models of SOD1-linked familial ALS. Trends Mol. Med. 2004;10:393–400. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous