The IL-33/ST2 pathway: therapeutic target and novel biomarker - PubMed (original) (raw)
Review
The IL-33/ST2 pathway: therapeutic target and novel biomarker
Rahul Kakkar et al. Nat Rev Drug Discov. 2008 Oct.
Abstract
For many years, the interleukin-1 receptor family member ST2 was an orphan receptor that was studied in the context of inflammatory and autoimmune disease. However, in 2005, a new cytokine--interleukin-33 (IL-33)--was identified as a functional ligand for ST2. IL-33/ST2 signalling is involved in T-cell mediated immune responses, but more recently, an unanticipated role in cardiovascular disease has been demonstrated. IL-33/ST2 not only represents a promising cardiovascular biomarker but also a novel mechanism of intramyocardial fibroblast-cardiomyocyte communication that may prove to be a therapeutic target for the prevention of heart failure.
Conflict of interest statement
Competing interests statement
The authors declare competing financial interests: see web version for details.
Figures
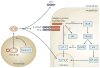
Figure 1. A model for IL-33/ST2 signalling
The myeloid differentiation factor 88 (MyD88)-dependent pathway of Toll-like receptor signalling involves Toll/Interleukin-1 receptor (TIR) dimerization between the receptor and the MyD88-adaptor-like protein (MAL). Recruitment of MyD88 and downstream activation of TNF receptor-associated factor 6 (TRAF6) via Interleukin-1 receptor-associated kinase (IRAK) proteins results in TRAF6-mediated activation of the inhibitor of nuclear factor-κB (
NF-κB
) kinase (IKK) complex and liberation of NF-κB from the complex. Free NF-κB is then able to bind DNA and act as a gene transcription regulator (reviewed in REF. 158). IL-33 signalling appears to share many of these properties and events downstream of IL-33 stimulation may include phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, p38 MAPK, JNKs as well as activation of NF-κB. It has been proposed that caspase-1-dependent cleavage of pro-IL-33, subsequent lysosomal navigation and fusion with the cell plasma membrane results in release of IL-33 into the interstitium as an active cytokine,. IL-33 binds to its receptor complex composed of ST2L (the transmembrane isoform of ST2) and the IL-1 receptor accessory protein IL-1RAcP. Subsequent sequestering of the adaptor proteins MyD88 and MAL results in modulation of IRAK mediated TRAF6 activation and subsequent mitogen-activated protein kinase (MAPK) and IKK/NF-κB activation,. The nature of this modulation of NF-κB activity by IL-33 is complex. In unstimulated cardiac myocytes and fibroblasts in vitro, exposure to IL-33 activates NF-κB. However, NF-κB activation via hypertrophic stimuli is attenuated by exposure to IL-33 (REF. 12). Interestingly, although TRAF6 appears to be required for IL-33-mediated NF-κB activation and downstream induction of Th2 cytokines, IL-33-mediated ERK activation might be TRAF6 independent. Furthermore, IL-33 might activate the transcription factor AP-1 independently of its effects on NF-κB. Exactly where the pivotal points of IL-33 signal regulation reside along this pathway from IL-33 receptor activation to NF-κB activity modulation is still unclear. Even before IL-33 binds to its receptor, its action could be altered by the decoy receptor soluble ST2 (sST2). sST2 is a variant of the full-length ST2 gene lacking the transmembrane and cytoplasmic domains contained within the structure of the transmembrane isoform of the gene. sST2 in the extracellular environment might bind free IL-33, thereby effectively decreasing the concentration of IL-33 that is available for ST2L binding and reducing the biological effect of IL-33 (REF. 12).
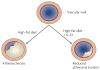
Figure 2. IL-33 in the type 2 immune response
Upon exposure to antigen and the proper interleukin milieu, CD4+ Th0 cells commit to either the Th1 or the Th2 lineage. As classically described, ‘type 1’ immune responses are typified by proliferation and activation of Th1 cells via exposure to certain interleukins including IL-12 and IL-18. Activated Th1 cells release characteristic cytokines such as IL-2 and interferon γ (INFγ) (among others) in response to pathogens. The ‘type 2’ response is typified by proliferation and activation of Th2 cells and release of their characteristic cytokines IL-4, IL-5 and IL-13 (among others) in response to extracellular, for example, parasitic, pathogens. How the appropriate immune response is chosen upon a particular threat has been the focus of much research and debate. Cells that first encounter invading pathogens (antigen-presenting cells) present foreign antigens to Th0 cells. Antigen presentation in combination with secretion of specific cytokines promotes the commitment of Th0 cells towards one lineage over another, and the initiation of a counter immune response to the infection (reviewed in REF. 165). In the presence of an antigen, direct stimulation of ST2L or exposure to IL-4 appears to be sufficient for the activation of Th2 cells and the release of Th2-associated cytokines. Exposure to IL-33 results in chemotaxis of Th2 cells and the release of Th2-associated cytokines. IL-33 can coax the release of Th2-associated cytokines from mast cells as well as basophils. IL-33 might also promote superoxide production and degranulation of eosinophils. Interestingly, recent evidence hints at a more promiscuous role for IL-33 as it has been found to induce INFγ release from antigen-exposed Th2 cells, natural killer (NK) cells and invariant NK T cells. To regulate this IL-33 mediated type 2 response, soluble ST2 (sST2) in the extracellular environment might act as a decoy, binding free IL-33 available to ST2L. Recently, it has been shown that a soluble form of the IL-1 receptor accessory protein (IL-1RAcP) might serve as a co-decoy, enhancing the ability of sST2 to inhibit IL-33 signalling.
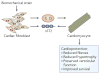
Figure 3. IL-33/ST2 signalling is a novel cardioprotective fibroblast–cardiomyocyte paracrine system
Any condition that alters the geometry or loading conditions of the left ventricle of the heart might alter the mechanical strain exerted on each individual cardiomyocyte. Myocytes are able to sense these changes in biomechanical strain and respond to them,,. Disease conditions that increase the stresses and strains on the ventricle, such as myocardial infarction, hypertension and valvular disease, result in hypertrophy of the myocytes and enhanced deposition of extracellular proteins (ventricular fibrosis), which, at least in the early adaptive phase of response, tends to normalize ventricular wall stress. These responses ultimately prove maladaptive, leading towards clinical heart failure–. The IL-33/ST2 system is emerging as a novel fibroblast–cardiomyocyte communication system that might abrogate these maladaptive processes. In response to biomechanical strain both cardiac myocytes and cardiac fibroblasts produce mature IL-33, although fibroblasts appear to be the dominant source. When in vitro cardiomyocytes subjected to hypertrophic signals were exposed to IL-33, the hypertrophic response was reduced. Addition of soluble ST2 (sST2) reversed this inhibition of hypertrophy, suggesting that it might serve as a decoy receptor. sST2 can be produced by both cardiac fibroblasts and cardiomyocytes. The ventricles of mice can be subjected to overt pressure overload by surgically constricting their aortae. In such a model, exposure to IL-33 reduced the normal ventricular hypertrophy and fibrosis that is seen as a consequence of the increased ventricular strain. Furthermore, the inevitable decrement in ventricular function and premature mortality noted in the mice subjected to ventricular pressure overload was reduced with IL-33 treatment.
Figure 4. IL-33/ST2 reduces atheroma formation
Atherosclerosis has been described as a chronic inflammatory disease of the vascular wall, characterized by a type I T-cell response to oxidized low density lipoprotein (LDL), as well as other antigens. It is this active inflammation that is thought to underlie the instability of some atherosclerotic lesions, leading to plaque rupture, subsequent clot formation, vessel lumen occlusion and the resultant downstream tissue infarction,. One strategy to inhibit the vascular inflammation of atherosclerosis might be to shift the balance towards Th2 immune-cell activation. The IL-33/ST2 system could be one pathway towards inducing this shift in balance. Mice lacking the gene for apolipoprotein E fed a high-fat diet have high serum cholesterol levels and develop atherosclerosis. When these mice are treated with IL-33, they display reduced aortic atherosclerotic plaque burden and lower levels of serum antibodies to oxidized LDL compared with control mice. Furthermore, when pre-treated with sST2 before IL-33 exposure, these mice display increased atherosclerosis compared with those not treated with soluble ST2; this is consistent with the anti-IL-33 effect of the soluble isoform of ST2.
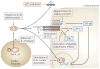
Figure 5. Strategies and consequences of IL-33/ST2 modulation
Stimulation of the IL-33/ST2 system might activate a cardioprotective programme in the context of ventricular biomechanical stress, and a number of approaches could be taken to modulate IL-33/ST2 signalling to capitalize on this cardioprotective activity. The IL-33 system may be activated by exogenous administration of IL-33 or by promoting IL-33 release from resident cardiac fibroblasts. Alternatively, availability of IL-33 to its receptor complex could be increased by inhibiting the IL-33 decoy receptor soluble ST2 (sST2). The system could also be activated by pharmacotherapeutics designed to directly stimulate the IL-33 receptor. Intracellular targeting may also be possible: Sequestration of the myeloid differentiation factor 88 (MyD88) by exogenous compounds might mimic the cardioprotective effects of IL-33 administration. If further clarity could be obtained regarding the nature and means by which IL-33 modulates nuclear factor-κB (NF-κB) activity, or with regard to the contribution of NF-κB-independent effects (such as direct stimulation of adaptor protein 1 (AP-1) or extracellular signal-regulated kinase (ERK)) to overall cardioprotection, these may also prove to be points along the IL-33 signalling cascade that are amenable to manipulation. Precisely how the nuclear and non-nuclear effects of IL-33 result in downstream cardioprotection is unclear. Further study into these processes could help identify novel methods of cardioprotection. Due to the involvement of the IL-33/ST2 system in a variety of processes, activation of this pathway may have unintended consequences. The involvement of IL-33 in Th2-mediated inflammation suggests that such a strategy might result in exacerbation of arthritic, asthmatic, rheumatologic and gastrointestinal inflammatory conditions. Conversely, inhibition of the IL-33/ST2 system to modulate these inflammatory conditions could result in increased cardiovascular injury in the face of ventricular strain. Care must be taken if the IL-33/ST2 system is to be manipulated for potential clinical gain.
Similar articles
- IL-33: a potential therapeutic target in autoimmune diseases.
Wang S, Ding L, Liu SS, Wang C, Leng RX, Chen GM, Fan YG, Pan HF, Ye DQ. Wang S, et al. J Investig Med. 2012 Dec;60(8):1151-6. doi: 10.2310/JIM.0b013e31826d8fcb. J Investig Med. 2012. PMID: 23076162 Review. - The potential role of IL-33/ST2 signaling in fibrotic diseases.
Gao Q, Li Y, Li M. Gao Q, et al. J Leukoc Biol. 2015 Jul;98(1):15-22. doi: 10.1189/jlb.3RU0115-012R. Epub 2015 Apr 16. J Leukoc Biol. 2015. PMID: 25881899 Review. - The IL-33/ST2 pathway--A new therapeutic target in cardiovascular disease.
Miller AM, Liew FY. Miller AM, et al. Pharmacol Ther. 2011 Aug;131(2):179-86. doi: 10.1016/j.pharmthera.2011.02.005. Epub 2011 Feb 26. Pharmacol Ther. 2011. PMID: 21356240 Review. - Research progress on interleukin-33 and its roles in the central nervous system.
Han P, Mi WL, Wang YQ. Han P, et al. Neurosci Bull. 2011 Oct;27(5):351-7. doi: 10.1007/s12264-011-1025-5. Neurosci Bull. 2011. PMID: 21934731 Free PMC article. Review.
Cited by
- DHT and Insulin Upregulate Secretion of the Soluble Decoy Receptor of IL-33 From Decidualized Endometrial Stromal Cells.
Salamon D, Ujvari D, Hellberg A, Hirschberg AL. Salamon D, et al. Endocrinology. 2023 Nov 20;165(1):bqad174. doi: 10.1210/endocr/bqad174. Endocrinology. 2023. PMID: 37972259 Free PMC article. - Suspected gut barrier disruptors and development of food allergy: Adjuvant effects and early immune responses.
Drønen EK, Namork E, Dirven H, Nygaard UC. Drønen EK, et al. Front Allergy. 2022 Nov 22;3:1029125. doi: 10.3389/falgy.2022.1029125. eCollection 2022. Front Allergy. 2022. PMID: 36483186 Free PMC article. - Measurement of multiple biomarkers in advanced stage heart failure patients treated with pulmonary artery catheter guided therapy.
Zilinski JL, Shah RV, Gaggin HK, Gantzer ML, Wang TJ, Januzzi JL. Zilinski JL, et al. Crit Care. 2012 Jul 25;16(4):R135. doi: 10.1186/cc11440. Crit Care. 2012. PMID: 22830581 Free PMC article. - IL-33 shifts macrophage polarization, promoting resistance against Pseudomonas aeruginosa keratitis.
Hazlett LD, McClellan SA, Barrett RP, Huang X, Zhang Y, Wu M, van Rooijen N, Szliter E. Hazlett LD, et al. Invest Ophthalmol Vis Sci. 2010 Mar;51(3):1524-32. doi: 10.1167/iovs.09-3983. Epub 2009 Nov 5. Invest Ophthalmol Vis Sci. 2010. PMID: 19892870 Free PMC article. - ST2 elevation in heart failure, predictive of a high early mortality.
Dalal JJ, Digrajkar A, Das B, Bansal M, Toomu A, Maisel AS. Dalal JJ, et al. Indian Heart J. 2018 Nov-Dec;70(6):822-827. doi: 10.1016/j.ihj.2018.08.019. Epub 2018 Aug 31. Indian Heart J. 2018. PMID: 30580851 Free PMC article.
References
- Sims JE. IL-1 and IL-18 receptors, and their extended family. Curr Opin Immunol. 2002;14:117–122. - PubMed
- Tominaga S. A putative protein of a growth specific cDNA from BALB/c-3T3 cells is highly similar to the extracellular portion of mouse interleukin 1 receptor. FEBS Lett. 1989;258:301–304. - PubMed
- Trajkovic V, Sweet MJ, Xu D. T1/ST2 — an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004;15:87–95. - PubMed
- Meisel C, et al. Regulation and function of T1/ST2 expression on CD4+ T cells: induction of type 2 cytokine production by T1/ST2 cross-linking. J Immunol. 2001;166:3143–3150. - PubMed
- Oshikawa K, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Resp Crit Care Med. 2001;164:277–281. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources