RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy - PubMed (original) (raw)
. 2008 Oct 1;68(19):7975-84.
doi: 10.1158/0008-5472.CAN-08-1401.
Swetlana Boldin-Adamsky, Rajesh K Thimmulappa, Srikanta K Rath, Hagit Ashush, Jonathan Coulter, Amanda Blackford, Steven N Goodman, Fred Bunz, Walter H Watson, Edward Gabrielson, Elena Feinstein, Shyam Biswal
Affiliations
- PMID: 18829555
- PMCID: PMC3070411
- DOI: 10.1158/0008-5472.CAN-08-1401
RNAi-mediated silencing of nuclear factor erythroid-2-related factor 2 gene expression in non-small cell lung cancer inhibits tumor growth and increases efficacy of chemotherapy
Anju Singh et al. Cancer Res. 2008.
Abstract
Nuclear factor erythroid-2-related factor 2 (Nrf2) is a redox-sensitive transcription factor that regulates the expression of electrophile and xenobiotic detoxification enzymes and efflux proteins, which confer cytoprotection against oxidative stress and apoptosis in normal cells. Loss of function mutations in the Nrf2 inhibitor, Kelch-like ECH-associated protein (Keap1), results in constitutive activation of Nrf2 function in non-small cell lung cancer. In this study, we show that constitutive activation of Nrf2 in lung cancer cells promotes tumorigenicity and contributes to chemoresistance by up-regulation of glutathione, thioredoxin, and the drug efflux pathways involved in detoxification of electrophiles and broad spectrum of drugs. RNAi-mediated reduction of Nrf2 expression in lung cancer cells induces generation of reactive oxygen species, suppresses tumor growth, and results in increased sensitivity to chemotherapeutic drug-induced cell death in vitro and in vivo. Inhibiting Nrf2 expression using naked siRNA duplexes in combination with carboplatin significantly inhibits tumor growth in a subcutaneous model of lung cancer. Thus, targeting Nrf2 activity in lung cancers, particularly those with Keap1 mutations, could be a promising strategy to inhibit tumor growth and circumvent chemoresistance.
Figures
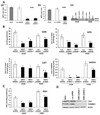
Figure. 1
(A) Generation of cell lines stably expressing Nrf2 shRNA. (a–c) Real time RT-PCR analysis of Nrf2 expression in A549 and H460 cells stably expressing Nrf2 shRNA. Total RNA from stable clones harboring Nrf2 shRNA or non-targeting luciferase shRNA were analyzed for expression of Nrf2. GAPDH was used as normalization control. (c) Immunoblot detection of Nrf2 in A549 and H460 cells stably transfected with shRNAs targeting Nrf2. (B–C) Comparison of GSR, GPX, GST, G6PDH enzyme activities and total GSH levels between cells expressing Nrf2 shRNA and control cells expressing luciferase shRNA. Data represent mean ± SE (n = 3). *, p < 0.05 relative to the cells expressing luciferase shRNA (by _t_-test). (D) Western blot analysis of TXN1 and TXNRD1 levels in A549 cells stably transfected with the Nrf2 shRNA and control cells expressing luciferase shRNA.
Figure. 2
Inhibition of Nrf2 activity leads to ROS accumulation in A549-Nrf2shRNA and H460-Nrf2shRNA cells. (A–B) Comparison of ROS levels in A549 and H460 cells stably expressing Nrf2 shRNA. Cells expressing non-targeting Luc shRNA were used as control. Pretreatment with 20mM NAC decreased the ROS levels. ROS levels in cells expressing luciferase shRNA were same as the control untransfected cells. (C) ROS levels did not change significantly between the BEAS2B cells transfected with Nrf2 siRNA and the control non-targeting NS siRNA. *, p < 0.01 relative to the cells expressing luciferase shRNA; **, p < 0.01 relative to the cells pretreated with NAC.
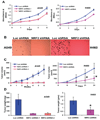
Figure. 3
Overexpression of Nrf2 confers drug resistance. (A–B) Effect of Nrf2 inhibition on drug accumulation in lung cancer cells. Tritium (3H) labeled etoposide and 14C labeled carboplatin accumulation in A549-Nrf2shRNA and H460-Nrf2shRNA cells was measured at regular time intervals (15–120 mins) after incubation with the drug. A non-targeting luciferase shRNA was used as control. Data are mean of 3 independent replicates, combined to generate the mean ± SE for each concentration. Drug accumulation was significantly higher in cells expressing Nrf2 shRNA. ‘*’, P<0.05 relative to Luc shRNA. (C–D) Enhanced sensitivity of A549-Nrf2shRNA and H460–Nrf2shRNA cells to carboplatin and etoposide. Cells were exposed to drugs for 72h– 96h and viable cells were determined by MTS/ phenazine methosulfate assay. Data is represented as percentage of viable cells relative to the vehicle treated control. Data are mean of 8 independent replicates, combined to generate the mean ± SD for each concentration. Representative experiments are shown.
Figure. 4
Nr2 ablation leads to reduced tumorigenic properties in vitro and in vivo. (A) Nrf2 promotes lung cancer cell proliferation. A549-Nrf2shRNA (1500 cells) and H460-Nrf2shRNA (1000 cells) cells were plated in 96 well plates and cellular proliferation was analyzed using the colorimetric MTS assay over the indicated time course. Cancer cells expressing Luc-shRNA were used as control. (B) A549-Nrf2shRNA and H460-Nrf2shRNA expressing cells were also analyzed for anchorage-independent growth. (C–D) A549-Nrf2shRNA and H460-Nrf2shRNA cells were injected in the flank of male athymic nude mice (n = 7 for H460, n=6 for A549). A549 and H460 cells expressing Luc-shRNA were used as control. Weekly measurements were taken from the tumors, and the mean tumor volume was determined after 4–6 weeks. Weight of the tumor was recorded at the termination of the experiment. Mean difference in tumor weight between the Luc-shRNA and Nrf2 shRNA expressing H460 cells was 1.24 gms (95% CI=0.773 to 1.71; “*” P=0.0001). Data was analyzed using two-sample Wilcoxon rank-sum (Mann-Whitney) test. A549-Nrf2 shRNA cells did not form any tumor in nude mice.
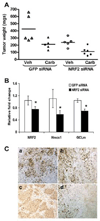
Figure. 5
Therapeutic efficacy of Nrf2 siRNA in combination with carboplatin. (A) Nude mice were injected subcutaneously with A549 cells and randomly allocated to one of the following groups with therapy beginning 15 days after tumor cell injection: GFP siRNA, GFP siRNA+ carboplatin, Nrf2 siRNA and Nrf2 siRNA+ carboplatin. Mice were treated for 4 weeks and then sacrificed. A dot plot shows the tumor weights upon termination by treatment group. Weights of the GFP siRNA treated tumors were significantly higher compared to Nrf2 siRNA treated tumors (ratio of weights = 2.09, 95% CI: [1.41, 3.10], p = 0.0002), and siRNA treated compared to siRNA+ carboplatin treated tumors (2.13, 95% CI: [1.44, 3.16], p = 0.001). (B) Delivery of naked Nrf2 siRNA duplex into tumor inhibited the expression of Nrf2 and its downstream target genes (HO-1 and GCLm). ‘*’,P<0.05 (Wilcoxon rank-sum test). (C) The proliferative index based on Ki-67 immunoreactivity in A549 tumors. Part “a”, shows large fraction of Ki-67 positive cells in GFP siRNA treated A549 tumors. Part ’b’ shows large number of Ki-67 stained cells in GFP siRNA+ carboplatin treated tumors. Part ‘c’ shows very few Ki-67 positive cells in Nrf2 siRNA treated tumors. Part ‘d’ shows ki-67 stained cells in Nrf2 siRNA+ carboplatin treated A549 tumors. Note that ‘d’ has area of extensive cell death (approximately the right half of the panel), and this massive cellular death was not seen in the other samples.
Similar articles
- Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer.
Singh A, Misra V, Thimmulappa RK, Lee H, Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E, Brock MV, Biswal S. Singh A, et al. PLoS Med. 2006 Oct;3(10):e420. doi: 10.1371/journal.pmed.0030420. PLoS Med. 2006. PMID: 17020408 Free PMC article. - Loss of Kelch-like ECH-associated protein 1 function in prostate cancer cells causes chemoresistance and radioresistance and promotes tumor growth.
Zhang P, Singh A, Yegnasubramanian S, Esopi D, Kombairaju P, Bodas M, Wu H, Bova SG, Biswal S. Zhang P, et al. Mol Cancer Ther. 2010 Feb;9(2):336-46. doi: 10.1158/1535-7163.MCT-09-0589. Epub 2010 Feb 2. Mol Cancer Ther. 2010. PMID: 20124447 Free PMC article. - A Novel Nrf2 Pathway Inhibitor Sensitizes Keap1-Mutant Lung Cancer Cells to Chemotherapy.
Zhang D, Hou Z, Aldrich KE, Lockwood L, Odom AL, Liby KT. Zhang D, et al. Mol Cancer Ther. 2021 Sep;20(9):1692-1701. doi: 10.1158/1535-7163.MCT-21-0210. Epub 2021 Jun 22. Mol Cancer Ther. 2021. PMID: 34158350 Free PMC article. - Emerging roles of Nrf2 signal in non-small cell lung cancer.
Tian Y, Liu Q, He X, Yuan X, Chen Y, Chu Q, Wu K. Tian Y, et al. J Hematol Oncol. 2016 Feb 27;9:14. doi: 10.1186/s13045-016-0246-5. J Hematol Oncol. 2016. PMID: 26922479 Free PMC article. Review. - Role of NRF2 in Lung Cancer.
Sánchez-Ortega M, Carrera AC, Garrido A. Sánchez-Ortega M, et al. Cells. 2021 Jul 24;10(8):1879. doi: 10.3390/cells10081879. Cells. 2021. PMID: 34440648 Free PMC article. Review.
Cited by
- Reduced mRNA expression levels of NFE2L2 are associated with poor outcome in breast cancer patients.
Wolf B, Goebel G, Hackl H, Fiegl H. Wolf B, et al. BMC Cancer. 2016 Oct 22;16(1):821. doi: 10.1186/s12885-016-2840-x. BMC Cancer. 2016. PMID: 27770790 Free PMC article. - CACUL1/CAC1 Regulates the Antioxidant Response by Stabilizing Nrf2.
Kigoshi Y, Fukuda T, Endo T, Hayasaka N, Iemura S, Natsume T, Tsuruta F, Chiba T. Kigoshi Y, et al. Sci Rep. 2015 Aug 4;5:12857. doi: 10.1038/srep12857. Sci Rep. 2015. PMID: 26238671 Free PMC article. - Reversal of multidrug resistance by cisplatin-loaded magnetic Fe3O4 nanoparticles in A549/DDP lung cancer cells in vitro and in vivo.
Li K, Chen B, Xu L, Feng J, Xia G, Cheng J, Wang J, Gao F, Wang X. Li K, et al. Int J Nanomedicine. 2013;8:1867-77. doi: 10.2147/IJN.S43752. Epub 2013 May 9. Int J Nanomedicine. 2013. PMID: 23690684 Free PMC article. - Arsenic inhibits autophagic flux, activating the Nrf2-Keap1 pathway in a p62-dependent manner.
Lau A, Zheng Y, Tao S, Wang H, Whitman SA, White E, Zhang DD. Lau A, et al. Mol Cell Biol. 2013 Jun;33(12):2436-46. doi: 10.1128/MCB.01748-12. Epub 2013 Apr 15. Mol Cell Biol. 2013. PMID: 23589329 Free PMC article. - Transcription factor NRF2 regulates miR-1 and miR-206 to drive tumorigenesis.
Singh A, Happel C, Manna SK, Acquaah-Mensah G, Carrerero J, Kumar S, Nasipuri P, Krausz KW, Wakabayashi N, Dewi R, Boros LG, Gonzalez FJ, Gabrielson E, Wong KK, Girnun G, Biswal S. Singh A, et al. J Clin Invest. 2013 Jul;123(7):2921-34. doi: 10.1172/JCI66353. Epub 2013 Jun 10. J Clin Invest. 2013. PMID: 23921124 Free PMC article.
References
- Nadkar A, Pungaliya C, Drake K, Zajac E, Singhal SS, Awasthi S. Therapeutic resistance in lung cancer. Expert Opin Drug Metab Toxicol. 2006;2:753–777. - PubMed
- Kurosu T, Fukuda T, Miki T, Miura O. BCL6 overexpression prevents increase in reactive oxygen species and inhibits apoptosis induced by chemotherapeutic reagents in B-cell lymphoma cells. Oncogene. 2003;22:4459–4468. - PubMed
- Masuda H, Tanaka T, Takahama U. Cisplatin generates superoxide anion by interaction with DNA in a cell-free system. Biochem Biophys Res Commun. 1994;203:1175–1180. - PubMed
- Soini Y, Napankangas U, Jarvinen K, Kaarteenaho-Wiik R, Paakko P, Kinnula VL. Expression of gamma-glutamyl cysteine synthetase in nonsmall cell lung carcinoma. Cancer. 2001;92:2911–2919. - PubMed
- Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 ES003819-17/ES/NIEHS NIH HHS/United States
- R01 HL081205/HL/NHLBI NIH HHS/United States
- P50 CA058184-13/CA/NCI NIH HHS/United States
- R01 HL081205-04/HL/NHLBI NIH HHS/United States
- P50 CA058184/CA/NCI NIH HHS/United States
- P30ES03819/ES/NIEHS NIH HHS/United States
- R01 CA104253-02/CA/NCI NIH HHS/United States
- R01 CA104253/CA/NCI NIH HHS/United States
- P30 ES003819/ES/NIEHS NIH HHS/United States
- P30 ES003819-20/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical