Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease - PubMed (original) (raw)
. 2008 Oct 7;105(40):15617-22.
doi: 10.1073/pnas.0805500105. Epub 2008 Oct 1.
Naina Bhasin, Claude Vieyres, Thomas J Hund, Shane R Cunha, Olha Koval, Celine Marionneau, Biyi Chen, Yuejin Wu, Sophie Demolombe, Long-Sheng Song, Hervé Le Marec, Vincent Probst, Jean-Jacques Schott, Mark E Anderson, Peter J Mohler
Affiliations
- PMID: 18832177
- PMCID: PMC2563133
- DOI: 10.1073/pnas.0805500105
Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease
Solena Le Scouarnec et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The identification of nearly a dozen ion channel genes involved in the genesis of human atrial and ventricular arrhythmias has been critical for the diagnosis and treatment of fatal cardiovascular diseases. In contrast, very little is known about the genetic and molecular mechanisms underlying human sinus node dysfunction (SND). Here, we report a genetic and molecular mechanism for human SND. We mapped two families with highly penetrant and severe SND to the human ANK2 (ankyrin-B/AnkB) locus. Mice heterozygous for AnkB phenocopy human SND displayed severe bradycardia and rate variability. AnkB is essential for normal membrane organization of sinoatrial node cell channels and transporters, and AnkB is required for physiological cardiac pacing. Finally, dysfunction in AnkB-based trafficking pathways causes abnormal sinoatrial node (SAN) electrical activity and SND. Together, our findings associate abnormal channel targeting with human SND and highlight the critical role of local membrane organization for sinoatrial node excitability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
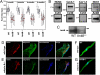
SND in human kindreds with ANK2 allele variants. (A) Family 1: Affected patients carry an AnkB-E1425G mutation depicted by a plus, whereas noncarriers are depicted by a minus. Other individuals did not undergo genetic testing. Note that at least 23 of 25 variant carriers (92%) display SND. (B) Family 2: Affected patients carry a common haplotype depicted by a black bar at the ANK2 locus. Markers D4S1572 and D4S427 delimitate the disease haplotype to a 16.5 cM interval (recombinations for patients III-9 and II-1, respectively). Squares represent males and circles represent females.
Fig. 2.
Ankyrin-B is expressed in human and mouse SAN and AnkB+/− mice display severe SND. (A) Adult AnkB− mice exhibit significant bradycardia and heart rate variability. Data represents mean ± SD for eight mice/genotype. (B) Immunoblots of human SAN tissue for SAN resident proteins HCN4, Cav3.1, Cav1.3, NCX1, and Cx45. Note that Cx43 is not a SAN-resident protein. We observed decreased expression of AnkB in all SAN preparations. (C) AnkB is expressed in SAN of the WT mouse heart and is significantly reduced in AnkB+/− adult mice. Equal protein loading was assessed by blotting for unrelated protein (data not shown, NHERF1). (D and E) AnkB is expressed in the mouse SAN. WT and AnkB+/− mouse sections were immunolabeled for AnkB and SAN markers HCN4 and neurofilament, and imaged by using identical protocols. E indicates loss of AnkB expression in AnkB+/− mice. (Scale bars, 10 μm.) (F and G) Expression of AnkB in isolated WT and AnkB+/− SAN cells. (Scale bars, 10 μm.)
Fig. 3.
NCX1, IP3R, Na/K ATPase, and Cav1.3 membrane expression is affected in AnkB+/− SAN cells. (A–P) Confocal imaging of SAN cells from WT and AnkB+/− mice. SAN cells were immunolabeled and imaged by using identical protocols. Note that NCX1 (A and B), Na/K ATPase (NKA) (M and N), and IP3R (O and P) immunolabeling is generally reduced across the cell, whereas Cav1.3 immunostaining is concentrated near the perinuclear region of AnkB+/− SAN cells (E and F). WT and AnkB+/− SAN cells displayed no difference in the expression or localization of Cav1.2 (C and D), Cav3.1 (G and H), HCN4 (I and J), RyR2 (K and L), or connexin 40 (data not shown). (Scale bars, 10 μm.)
Fig. 4.
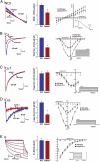
Reduced INCX and ICa,L in AnkB+/− SAN cells. Reduction of AnkB leads to reduced NCX1 and L-type Ca2+ currents. (A) INCX density is significantly lower in isolated AnkB+/− SAN cells compared to WT at voltages greater than 0 mV (n = 12, P < 0.05). Raw trace and bar graph represent current at −10 mV. (B) ICa density is reduced significantly in isolated AnkB+/− SAN cells compared to WT cells at all voltages tested (n = 10, P < 0.05). Raw trace and bar graph represent current at −10 mV. (C and D) T-type Ca2+ current is unchanged between WT and AnkB+/− SAN cells (n = 10, NS), whereas L-type Ca2+ current is dramatically reduced in AnkB+/− SAN cells (n = 10, P < 0.05). Raw traces and bar graphs represent current at −20 mV (ICa,T) and 0 mV (_I_Ca,L). (E) WT and AnkB+/− SAN cells display similar _I_f current (n = 10, NS). Bar graph represents current at −80 mV.
Fig. 5.
AnkB is required for SAN Ca2+ homeostasis. (A) Rate and frequency of Ca2+ transients from isolated SAN cells of WT and AnkB+/− mice were measured by using Fluo-3 AM and confocal imaging. Note reduced rate and extreme rate variability in AnkB+/− SAN cells. (B) Fourier transformation of pooled data from eight independent experiments from rate and frequency measurements in A. Note that AnkB+/− SAN explants display increased power density at ≥2 dominant frequencies. (C) Ca2+ transients of WT and AnkB+/− mouse SAN. AnkB+/− cells display increased cycle length and inconsistent response to isoproterenol. (D) Mean data from isoproterenol experiments. (n = 8 per genotype, P < 0.05.)
Similar articles
- Ankyrin-based targeting pathway regulates human sinoatrial node automaticity.
Hund TJ, Mohler PJ. Hund TJ, et al. Channels (Austin). 2008 Nov-Dec;2(6):404-6. doi: 10.4161/chan.2.6.7220. Epub 2008 Nov 20. Channels (Austin). 2008. PMID: 19098452 - Functional anatomy of the murine sinus node: high-resolution optical mapping of ankyrin-B heterozygous mice.
Glukhov AV, Fedorov VV, Anderson ME, Mohler PJ, Efimov IR. Glukhov AV, et al. Am J Physiol Heart Circ Physiol. 2010 Aug;299(2):H482-91. doi: 10.1152/ajpheart.00756.2009. Epub 2010 Jun 4. Am J Physiol Heart Circ Physiol. 2010. PMID: 20525877 Free PMC article. - Atrial fibrillation and sinus node dysfunction in human ankyrin-B syndrome: a computational analysis.
Wolf RM, Glynn P, Hashemi S, Zarei K, Mitchell CC, Anderson ME, Mohler PJ, Hund TJ. Wolf RM, et al. Am J Physiol Heart Circ Physiol. 2013 May;304(9):H1253-66. doi: 10.1152/ajpheart.00734.2012. Epub 2013 Feb 22. Am J Physiol Heart Circ Physiol. 2013. PMID: 23436330 Free PMC article. - Ankyrins and human disease: what the electrophysiologist should know.
Mohler PJ. Mohler PJ. J Cardiovasc Electrophysiol. 2006 Oct;17(10):1153-9. doi: 10.1111/j.1540-8167.2006.00540.x. Epub 2006 Jun 27. J Cardiovasc Electrophysiol. 2006. PMID: 16800854 Review. - Cardiac ankyrins in health and disease.
Hashemi SM, Hund TJ, Mohler PJ. Hashemi SM, et al. J Mol Cell Cardiol. 2009 Aug;47(2):203-9. doi: 10.1016/j.yjmcc.2009.04.010. Epub 2009 Apr 24. J Mol Cell Cardiol. 2009. PMID: 19394342 Free PMC article. Review.
Cited by
- Use of whole exome sequencing for the identification of Ito-based arrhythmia mechanism and therapy.
Sturm AC, Kline CF, Glynn P, Johnson BL, Curran J, Kilic A, Higgins RS, Binkley PF, Janssen PM, Weiss R, Raman SV, Fowler SJ, Priori SG, Hund TJ, Carnes CA, Mohler PJ. Sturm AC, et al. J Am Heart Assoc. 2015 May 26;4(5):e001762. doi: 10.1161/JAHA.114.001762. J Am Heart Assoc. 2015. PMID: 26015324 Free PMC article. - Defects in cytoskeletal signaling pathways, arrhythmia, and sudden cardiac death.
Smith S, Curran J, Hund TJ, Mohler PJ. Smith S, et al. Front Physiol. 2012 May 3;3:122. doi: 10.3389/fphys.2012.00122. eCollection 2012. Front Physiol. 2012. PMID: 22586405 Free PMC article. - Two-Pore K+ Channel TREK-1 Regulates Sinoatrial Node Membrane Excitability.
Unudurthi SD, Wu X, Qian L, Amari F, Onal B, Li N, Makara MA, Smith SA, Snyder J, Fedorov VV, Coppola V, Anderson ME, Mohler PJ, Hund TJ. Unudurthi SD, et al. J Am Heart Assoc. 2016 Apr 20;5(4):e002865. doi: 10.1161/JAHA.115.002865. J Am Heart Assoc. 2016. PMID: 27098968 Free PMC article. - The cardiac conduction system.
Park DS, Fishman GI. Park DS, et al. Circulation. 2011 Mar 1;123(8):904-15. doi: 10.1161/CIRCULATIONAHA.110.942284. Circulation. 2011. PMID: 21357845 Free PMC article. Review. No abstract available. - Arrhythmogenic Cardiomyopathy: Molecular Insights for Improved Therapeutic Design.
Stevens TL, Wallace MJ, Refaey ME, Roberts JD, Koenig SN, Mohler PJ. Stevens TL, et al. J Cardiovasc Dev Dis. 2020 May 26;7(2):21. doi: 10.3390/jcdd7020021. J Cardiovasc Dev Dis. 2020. PMID: 32466575 Free PMC article. Review.
References
- Mangrum JM, DiMarco JP. The evaluation and management of bradycardia. N Engl J Med. 2000;342:703–709. - PubMed
- Kusumoto FM, Goldschlager N. Cardiac pacing. N Engl J Med. 1996;334:89–97. - PubMed
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;55:455–472. - PubMed
- Dobrzynski H, Boyett MR, Anderson RH. New insights into pacemaker activity: Promoting understanding of sick sinus syndrome. Circulation. 2007;115:1921–1932. - PubMed
- Lamas GA, et al. The mode selection trial (MOST) in sinus node dysfunction: Design, rationale, and baseline characteristics of the first 1,000 patients. Am Heart J. 2000;140:541–551. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL084583/HL/NHLBI NIH HHS/United States
- HL62494/HL/NHLBI NIH HHS/United States
- R01 HL062494/HL/NHLBI NIH HHS/United States
- R01 HL079031/HL/NHLBI NIH HHS/United States
- HL70250/HL/NHLBI NIH HHS/United States
- R01 HL083422/HL/NHLBI NIH HHS/United States
- HL083422/HL/NHLBI NIH HHS/United States
- HL079031/HL/NHLBI NIH HHS/United States
- R01 HL084583/HL/NHLBI NIH HHS/United States
- R01 HL070250/HL/NHLBI NIH HHS/United States
- HL090905/HL/NHLBI NIH HHS/United States
- R01 HL090905/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases