Ribosomal protein L3 functions as a 'rocker switch' to aid in coordinating of large subunit-associated functions in eukaryotes and Archaea - PubMed (original) (raw)
Ribosomal protein L3 functions as a 'rocker switch' to aid in coordinating of large subunit-associated functions in eukaryotes and Archaea
Arturas Meskauskas et al. Nucleic Acids Res. 2008 Nov.
Abstract
Although ribosomal RNAs (rRNAs) comprise the bulk of the ribosome and carry out its main functions, ribosomal proteins also appear to play important structural and functional roles. Many ribosomal proteins contain long, nonglobular domains that extend deep into the rRNA cores. In eukaryotes and Archaea, ribosomal protein L3 contains two such extended domains tethered to a common globular hub, thus providing an excellent model to address basic questions relating to ribosomal protein structure/function relationships. Previous work in our laboratory identified the central 'W-finger' extension of yeast L3 in helping to coordinate ribosomal functions. New studies on the 'N-terminal' extension in yeast suggest that it works with the W-finger to coordinate opening and closing of the corridor through which the 3' end of aa-tRNA moves during the process of accommodation. Additionally, the effect of one of the L3 N-terminal extension mutants on the interaction between C75 of the aa-tRNA and G2921 (Escherichia coli G2553) of 25S rRNA provides the first evidence of the effect of a ribosomal protein on aa-tRNA positioning and peptidyltransfer, possibly through the induced fit model. A model is presented describing how all three domains of L3 may function together as a 'rocker switch' to coordinate the stepwise processes of translation elongation.
Figures
Figure 1.
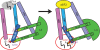
The N-terminal extension of ribosomal protein L3. (A) The globular domain of ribosomal protein L3 (green) abuts the base of helix 95. The elongation factor binding site is at the top of helices 89, 91 and 95, and the aa-tRNA accommodation corridor is between Helices 91 and 89. The peptidyltransferase center (E. coli A2451, H. marismortui A2484, yeast A2819), lies at the bottom of this corridor and is colored red. The W-finger of L3 extends to under the base of Helix 90, on the A site side of the peptidyltransferase center. The _N_-terminal extension of L3 (denoted as pink spheres) extends into a complex structure formed by Helices 90 and 92. (B) Close-up view of the _N_-terminus of L3 nestled between Helices 90 and 92. (C) Ten-fold dilution spot assays of mutants in the N-terminal extension of L3. The serine at the _N_-terminus of L3 (S2) was mutated to the indicated amino acids. In iG12, an alanine was inserted between histidine at position 11 and the glycine at position 12. ΔG12 indicates deletion of glycine at position 12.
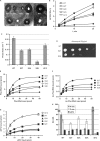
Figure 2.
Genetic and biochemical characterization of ribosomes and cells expressing the L3 N-terminal extension mutants. (A) Killer virus maintenance profiles of L3 N-terminal mutants. Presence of, and relative amount of intracellular killer virus is indicated by the diameter of the zone of growth inhibition around the indicated colonies. (B and C) First-order time plots of Ac-[14C]Phe-puromycin formation were used to calculate observed rates of peptidyltransferase activity (_K_obs). (D) Anisomycin resistance phenotypes of S2T and S2K mutants. Ten-fold dilutions of cells harboring the indicated rpl3 alleles were spotted onto complete synthetic medium lacking tryptophan containing anisomycin (50 μg/ml), and were incubated at 30°C for 3 days. (E) Single site isothems of eEF-1A stimulated binding of [14C]Phe-tRNA to A-sites of poly(U) primed ribosomes. (F) Binding isotherms of Ac-[14C]Phe-tRNA to P-sites of poly(U) primed ribosomes. (G) eEF2 binding isotherms for wild-type and mutant ribosomes. (H) Dissociation constants were calculated from data shown in panels E, F and G. Error bars denote standard deviations. N.D. denotes not determined.
Figure 3.
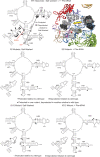
rRNA structure probing of wild-type and the L3 N-terminal extension mutant ribosomes. Autoradiograms at left are of salt-washed wild-type, S2K, S2T, S2A and iG12 mutant ribosomes, while those at right are of nonsalt-washed ribosomes loaded with aa-tRNA. Ribosomes were unmodified or treated with DMS, CMCT or kethoxal as indicated. Reverse transcriptase primer extension reactions spanned sequences in Helix 89 (top panels), Helices 90–92 (ASL, middle panels) and Helix 95 (SRL, lower panels). Sequencing reactions (left sides of panels) are labeled corresponding to the rRNA sense strand. Bases in wild-type ribosomes loaded with aa-tRNAs that are deprotected from chemical attack relative to salt-washed ribosomes are indicated by black arrowheads. Similarly, those that become protected upon addition of aa-tRNA are indicated by white arrowheads. Bases in mutant ribosomes that are deprotected relative to wild-type are indicated by black circles, and those that are hyperprotected are indicated by white circles.
Figure 4.
rRNA protection of the S2 mutants mapped onto yeast 25S rRNA and the _H. marismortui/_yeast large subunit structure. (Top) Left panel shows yeast 25S rRNA bases whose chemical protection patterns change in the presence of aa-tRNA in wild-type ribosomes. Base numbering follows the S. cerevisiae sequence. Black arrows indicate deprotection, and white arrows show hyperprotection in Phe-tRNA containing ribosomes as compared to salt-washed ribosomes. The positions of bases involved in formation of the two aa-tRNA accommodation corridor ‘gates’ (48) are indicated, as well as bases that interact with S2 and W255. In the right panel, these bases have been mapped onto the H. marismortui/yeast structure. Ribosomal protein L3 is colored green, and L10 is in red. Black and gray spheres indicate deprotected and hyperprotected bases, respectively. Yellow arrow connotes movement of Helix 91–92 structure toward Helix 89 to close the accommodation corridor. (Center) Bases of 25S rRNA in the S2 series of mutants whose modification patterns differ from wild-type ribosomes. Left and right panels map changes in salt-washed and aa-tRNA loaded ribosomes. Black and white circles indicate deprotected and hyperprotected bases, respectively. (Bottom) Bases of 25S rRNA in the iG12 mutant whose modification patterns differ from wild-type ribosomes. Left and right panels map changes in salt-washed and aa-tRNA loaded ribosomes. Black and white circles indicate deprotected and hyperprotected bases, respectively.
Figure 5.
L3 functions as a ‘rocker switch’ to coordinate elongation factor binding, aa-tRNA accommodation and PTC activation. (Left) Ribosome in ground state with P site occupied by peptidyl-tRNA. L3 W-finger is in the ‘extended’ conformation, maintaining the A site in the closed conformation. Interaction between H259 of L3 and A2940 (E. coli A2572) of 25S rRNA stabilize this state. The L3 N-terminal extension is in the ‘retracted’ conformation, opening the accommodation corridor. (Right) accommodation of aa-tRNA into the A site leads to the following events. (A) Opening of A site and retraction of the W-finger, stabilized by interaction between W255 of L3 and A2940 of 25S rRNA. (B) Rotation of L3 globular domain. (C) Extension of the N-terminal domain toward Helix 89. (D) Displacement of Helix 91, and closure of the accommodation corridor. (E) Repositioning of the H90/H92 structure and of G2921 (E. coli G2553) in the A site, where it can interact with C75 of the aa-tRNA to activate peptidyltransfer (55). (F) Movement of H91 away from H95 results in formation of the eEF2-binding site.
Similar articles
- A molecular clamp ensures allosteric coordination of peptidyltransfer and ligand binding to the ribosomal A-site.
Meskauskas A, Dinman JD. Meskauskas A, et al. Nucleic Acids Res. 2010 Nov;38(21):7800-13. doi: 10.1093/nar/gkq641. Epub 2010 Jul 21. Nucleic Acids Res. 2010. PMID: 20660012 Free PMC article. - Ribosomal protein L3: gatekeeper to the A site.
Meskauskas A, Dinman JD. Meskauskas A, et al. Mol Cell. 2007 Mar 23;25(6):877-88. doi: 10.1016/j.molcel.2007.02.015. Mol Cell. 2007. PMID: 17386264 Free PMC article. - Yeast ribosomal protein L10 helps coordinate tRNA movement through the large subunit.
Petrov AN, Meskauskas A, Roshwalb SC, Dinman JD. Petrov AN, et al. Nucleic Acids Res. 2008 Nov;36(19):6187-98. doi: 10.1093/nar/gkn643. Epub 2008 Sep 29. Nucleic Acids Res. 2008. PMID: 18824477 Free PMC article. - Assembling the archaeal ribosome: roles for translation-factor-related GTPases.
Blombach F, Brouns SJ, van der Oost J. Blombach F, et al. Biochem Soc Trans. 2011 Jan;39(1):45-50. doi: 10.1042/BST0390045. Biochem Soc Trans. 2011. PMID: 21265745 Review. - Structure of a two-domain N-terminal fragment of ribosomal protein L10 from Methanococcus jannaschii reveals a specific piece of the archaeal ribosomal stalk.
Kravchenko O, Mitroshin I, Nikonov S, Piendl W, Garber M. Kravchenko O, et al. J Mol Biol. 2010 Jun 4;399(2):214-20. doi: 10.1016/j.jmb.2010.04.017. Epub 2010 Apr 24. J Mol Biol. 2010. PMID: 20399793 Review.
Cited by
- A molecular clamp ensures allosteric coordination of peptidyltransfer and ligand binding to the ribosomal A-site.
Meskauskas A, Dinman JD. Meskauskas A, et al. Nucleic Acids Res. 2010 Nov;38(21):7800-13. doi: 10.1093/nar/gkq641. Epub 2010 Jul 21. Nucleic Acids Res. 2010. PMID: 20660012 Free PMC article. - Trichothecenes in Cereal Grains - An Update.
Foroud NA, Baines D, Gagkaeva TY, Thakor N, Badea A, Steiner B, Bürstmayr M, Bürstmayr H. Foroud NA, et al. Toxins (Basel). 2019 Oct 31;11(11):634. doi: 10.3390/toxins11110634. Toxins (Basel). 2019. PMID: 31683661 Free PMC article. Review. - Ribosomes in the balance: structural equilibrium ensures translational fidelity and proper gene expression.
Musalgaonkar S, Moomau CA, Dinman JD. Musalgaonkar S, et al. Nucleic Acids Res. 2014 Dec 1;42(21):13384-92. doi: 10.1093/nar/gku1020. Epub 2014 Nov 11. Nucleic Acids Res. 2014. PMID: 25389262 Free PMC article. - The central core region of yeast ribosomal protein L11 is important for subunit joining and translational fidelity.
Rhodin MH, Rakauskaitė R, Dinman JD. Rhodin MH, et al. Mol Genet Genomics. 2011 Jun;285(6):505-16. doi: 10.1007/s00438-011-0623-2. Epub 2011 Apr 26. Mol Genet Genomics. 2011. PMID: 21519857 Free PMC article. - mRNA decoding in human is kinetically and structurally distinct from bacteria.
Holm M, Natchiar SK, Rundlet EJ, Myasnikov AG, Watson ZL, Altman RB, Wang HY, Taunton J, Blanchard SC. Holm M, et al. Nature. 2023 May;617(7959):200-207. doi: 10.1038/s41586-023-05908-w. Epub 2023 Apr 5. Nature. 2023. PMID: 37020024 Free PMC article.
References
- Spirin AS. The ribosome as an RNA-based molecular machine. RNA Biol. 2004;1:3–9. - PubMed
- Yusupova G, Jenner L, Rees B, Moras D, Yusupov M. Structural basis for messenger RNA movement on the ribosome. Nature. 2006;444:391–394. - PubMed
- Noller HF. Structure of the bacterial ribosome and some implications for translational regulation. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2007. pp. 41–58.
- Mitra K, Frank J. Ribosome dynamics: insights from atomic structure modeling into cryo-electron microscopy maps. Annu. Rev. Biophys. Biomol. Struct. 2006;35:299–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases