Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates - PubMed (original) (raw)
Zebrafish eda and edar mutants reveal conserved and ancestral roles of ectodysplasin signaling in vertebrates
Matthew P Harris et al. PLoS Genet. 2008.
Abstract
The genetic basis of the development and variation of adult form of vertebrates is not well understood. To address this problem, we performed a mutant screen to identify genes essential for the formation of adult skeletal structures of the zebrafish. Here, we describe the phenotypic and molecular characterization of a set of mutants showing loss of adult structures of the dermal skeleton, such as the rays of the fins and the scales, as well as the pharyngeal teeth. The mutations represent adult-viable, loss of function alleles in the ectodysplasin (eda) and ectodysplasin receptor (edar) genes. These genes are frequently mutated in the human hereditary disease hypohidrotic ectodermal dysplasia (HED; OMIM 224900, 305100) that affects the development of integumentary appendages such as hair and teeth. We find mutations in zebrafish edar that affect similar residues as mutated in human cases of HED and show similar phenotypic consequences. eda and edar are not required for early zebrafish development, but are rather specific for the development of adult skeletal and dental structures. We find that the defects of the fins and scales are due to the role of Eda signaling in organizing epidermal cells into discrete signaling centers of the scale epidermal placode and fin fold. Our genetic analysis demonstrates dose-sensitive and organ-specific response to alteration in levels of Eda signaling. In addition, we show substantial buffering of the effect of loss of edar function in different genetic backgrounds, suggesting canalization of this developmental system. We uncover a previously unknown role of Eda signaling in teleosts and show conservation of the developmental mechanisms involved in the formation and variation of both integumentary appendages and limbs. Lastly, our findings point to the utility of adult genetic screens in the zebrafish in identifying essential developmental processes involved in human disease and in morphological evolution.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
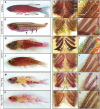
Figure 1. The formation of the adult dermal skeleton and pharyngeal teeth is affected in fls mutant zebrafish.
A) Alizarin red-stained wild type adult zebrafish shows staining of the scales, fin rays, dermal bones of the skull as well as the pharyngeal teeth along ceratobranchial 5 (bracket, B) and (C) gill rakers along both the anterior (antGr) and posterior edge (postGr) of non-teeth bearing ceratobranchials. D) fls te370f shows loss of dermal skeletal structures of the fin rays, scales and alteration in the shape of the skull. Additionally, fls te370f shows loss of pharyngeal teeth (E) and gill rakers (F). G–I) The Topless allele (flsdt3Tpl) shows a dominant effect on scalation and tooth/gill raker formation while not affecting lepidotrichial growth. J–L) Expressivity of fls dt3Tpl is sensitive to a modifier in the Tü strain leading to a “weak” fls dt3Tpl phenotype; fls dt3Tpl homozygotes were phenotypically identical to fls te370f (not shown). The fang allele of fls, fls tfang, isolated in a non-complementation screen with fls te370f, shows no effect on fin development while exhibiting partial loss of scales (M), teeth (N), and gill rakers (O). Transallelic tfang/te370f zebrafish exhibit an intermediate phenotype between homozygous fls(te370f) and fls(tfang) (P–R).
Figure 2. The dominant gene Nkt is phenotypically similar, however complements fls mutants.
Nkt homozygotes show complete loss of scales, teeth and gill rakers resembling the fls phenotype (A–C). Heterozygous Nkt zebrafish show an intermediate phenotype of scale loss and patterning defect (arrows) while no effect on fin development is seen (D). Heterozygous Nkt also show a dominant effect on the number of teeth (arrows, E) and gill rakers (F), showing deficiencies along the posterior branchial arches and formation of rudimentary rakers along ceratobranchial 1 and 2 (arrows, F). Cb1-5, ceratobranchial bones.
Figure 3. fls and Nkt are mutations in genes encoding ectodysplasin receptor (edar) and its ligand ectodysplasin (eda).
A) Mapping of fls using SSLP markers and placement of the edar gene within the candidate region on LG 9 by radiation hybrid mapping. The insert shows genetic linkage of the fls gene to local markers on LG 9. The numbers on the right of the insert indicate the number of recombinants seen in identified mutants per the number of meioses tested. B) Schematic of wild type Edar protein and mutant alleles. Polymorphisms seen in the WIK strain are noted above the wild type gene. Mutations that lead to premature termination are represented as truncated proteins showing the predicted residual fragment and position of the identified mutation. C) Analysis of the mutation in fls dfang. A unique splice donor site (red) is generated leading to inclusion of additional coding sequence encoding a premature termination codon (underlined). D) Quantitative analysis of different edar transcript levels in fls dfang homozygotes compared with wildtype. E) Similarity of altered residues in fls t3R367Wand fls dt3Tpl with human HED shown in the death domain. The position of the mutated residues in fls t3R367W (blue box) and in fls dt3Tpl (red box) is identical to ones changed in cases of human autosomal dominant HED although the substitution is different. F) Linkage between the Nkt allele and eda on LG5. G) Schematic of wild type Eda protein and position of Nkt mutation. Numbers on gene diagrams represent amino acid length. TNF, tumor necrosis factor domain; TNFR, tumor necrosis factor receptor domain; TM, transmembrane domain; DD, death domain; the blue box in Eda is the furin binding site.
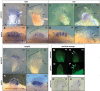
Figure 4. The role of edar in expression of developmental patterning genes during early scale development.
Expression of edar (A, B) and eda (C, D) in early forming scales; arrowheads indicate site of expression of initial forming scales. A) edar expression above site of scale formation in 8 mm long (approximately 30 dpf juvenile fish) and in larger juveniles (9 mm; 30 dpf). C) eda expression during early scale development on the flank (8 mm) and in forming scales of older juvenile fish (10 mm, D). Expression of developmental genes bmp2b and shh in early scale development in wildtype (E, G) and fls te370f (F, H) juveniles (9 mm).
Figure 5. Eda signaling regulates the formation of an epidermal placode during scale development.
Histological analysis of wild type (A, D, B, E) and fls te370f (C, F, I) integument of 8 mm standard length. In wild type juveniles (B, E), basal epidermal cells (black arrow heads) show a heightened, and cuboidal morphology at sites of scale development as indicated by an accumulation of migrating fibroblast-like cells (white arrowheads). (H) This morphology of the epidermis is associated with a reworking of the collagen layer of the stratum compactum (cpt; [24]). This is in contrast to the flattened morphology of basal epidermal cells lateral to those of the scale placode (A, D) and underlying dense stratum compactum (G). In fls te370f this basal epidermal structure is disorganized and cell morphology is disrupted (C, F) including evidence of cell death (asterisk). The lack of reworking of the collagen of the stratum compactum in the fls te370f mutant is associated with retention of hemidesmosomes (horizontal bracket G–I). edar is expressed in cells of the wildtype epidermis (J, K, L). Counterstaining of the same sections confirms the expression in basal cells overlying initial accumulating fibroblasts (white arrowheads; J′, K′, L′). Expression of edar is observed prior to organization of the placode and fibroblast aggregation and maintained in cells of the epidermal placode through early scale development (J–L). M) Schematic depicting scale development and edar expression. The stages of scale development are modeled using analogous stages as described for hair development ; stage 0, nascent epidermis; stage 1, placode specification; stage 2, scale pocket; stage 3, matrix deposition and ossification. Blue, edar expression; red, scale formation. ep, epidermis; cpt stratum compactum. The vertical bracket demarcates the extent of the epidermis in the sections. Measurement bar equals 10 μm.
Figure 6. Fin development is defective in fls and Nkt mutant zebrafish.
Alizarin red stained adult zebrafish fins show a drastic effect of fls te370f and Nkt on development of the lepidotrichial dermal rays of both the paired and unpaired fins. A–C,F) Pectoral fins, anterior-dorsal view; D, E) double staining developing pectoral fins with alcian blue and alizarin red show early patterning of the endochondrial bones of the pectoral fin of size matched wild type and Nkt homozygotes (asterisk indicates loss of fourth proximal radial). G) effect of fls and Nkt mutants on the patterning of the pectoral fin skeleton scored as the number of specimens showing alteration in pattern or form over total analyzed. The identity of the proximal radials is noted (I–IV). H–J) Analysis of pelvic fin development in fls and Nkt mutants. Numbers denote anterior-posterior identity of the lepidotrichia. K–M) Defects in the formation of the lepidotrichia in adult anal and (N–P) caudal fin of fls (L and O) and Nkt (M and P). ap, ascending process; cl, cleithrum; co, corticoid; dr, distal radial; sc, scalpula, le, lepidotrichia; pcl, postcleithrum; pr, proximal radial.
Figure 7. Eda signaling and the maintenance of anterior-posterior pattern in late paired fin development.
Analysis of edar (A–B, E–F), shh (C–D, G–H), and bmp2b (I–L) expression in developing pectoral (A–D, –J) and pelvic fins (E–H, K–L) from 8 mm juvenile fish of wild type (A, E; C, G; I, K) and fls te370f mutant fish (B, F; D, H; J, L). A–B) Arrowheads indicate two distinct patterns of edar expression in the pectoral fin: an expression that marks the posterior edge and distal region of the development of the proximal radials (black); and a posterior bias of edar expression in the forming lepidotrichia (white). Arrows point out the remaining posterior expression in mutant fins. Brackets in all panels outline anterior deficiencies in gene expression in fls mutant fins. M–P, analysis of patterns of cell death in the developing paired fins by retention of acridine orange stain. N, P) Arrows point out anterior distal regions of cell death in both pectoral and pelvic fins from the mutant; (M, N) pectoral fin and (O, P) pelvic fin respectively. Q, R) Expression of msxa in wild type and mutant pectoral fins. Region of expression outlined with brackets; asterisk marks an ectopic site of expression.
Figure 8. Eda signaling is required for the function of the fin fold during late fin development.
Expression of bmp2b (A), shh (B) and edar (C) transcripts in developing juvenile (8 mm, 30 dpf) fin rays of the caudal fin; asterisks indicate regional expression within distal tip of developing ray; arrows in (C), expression in distal epidermis of the fin fold. D–I) Histological analysis of both paired (pectoral, D, E) and unpaired fins (anal F, G; and caudal, H, I) from fls te370f and wild type siblings. fls te370f fins showed a general deficiency in the maturation of the muscle and dermis of the fin (arrow G; acellular debris in anal fin of fls). Insets (D, E), tip of fin at higher magnification showing degeneration of the nuclei of the epidermis in the mutant fin. ffd, fin fold; le, lepidotrichia of the fin rays.
Similar articles
- Eda/Edar signaling guides fin ray formation with preceding osteoblast differentiation, as revealed by analyses of the medaka all-fin less mutant afl.
Iida Y, Hibiya K, Inohaya K, Kudo A. Iida Y, et al. Dev Dyn. 2014 Jun;243(6):765-77. doi: 10.1002/dvdy.24120. Epub 2014 Mar 19. Dev Dyn. 2014. PMID: 24585696 - Gene Mutations of the Three Ectodysplasin Pathway Key Players (EDA, EDAR, and EDARADD) Account for More than 60% of Egyptian Ectodermal Dysplasia: A Report of Seven Novel Mutations.
Ahmed HA, El-Kamah GY, Rabie E, Mostafa MI, Abouzaid MR, Hassib NF, Mehrez MI, Abdel-Kader MA, Mohsen YH, Zada SK, Amr KS, Sayed ISM. Ahmed HA, et al. Genes (Basel). 2021 Sep 8;12(9):1389. doi: 10.3390/genes12091389. Genes (Basel). 2021. PMID: 34573371 Free PMC article. - Ectodysplasin receptor-mediated signaling is essential for embryonic submandibular salivary gland development.
Jaskoll T, Zhou YM, Trump G, Melnick M. Jaskoll T, et al. Anat Rec A Discov Mol Cell Evol Biol. 2003 Apr;271(2):322-31. doi: 10.1002/ar.a.10045. Anat Rec A Discov Mol Cell Evol Biol. 2003. PMID: 12629675 - Molecular aspects of hypohidrotic ectodermal dysplasia.
Mikkola ML. Mikkola ML. Am J Med Genet A. 2009 Sep;149A(9):2031-6. doi: 10.1002/ajmg.a.32855. Am J Med Genet A. 2009. PMID: 19681132 Review. - Ectodysplasin research--where to next?
Lefebvre S, Mikkola ML. Lefebvre S, et al. Semin Immunol. 2014 Jun;26(3):220-8. doi: 10.1016/j.smim.2014.05.002. Epub 2014 Jun 10. Semin Immunol. 2014. PMID: 24928340 Review.
Cited by
- Re-epithelialization of cutaneous wounds in adult zebrafish combines mechanisms of wound closure in embryonic and adult mammals.
Richardson R, Metzger M, Knyphausen P, Ramezani T, Slanchev K, Kraus C, Schmelzer E, Hammerschmidt M. Richardson R, et al. Development. 2016 Jun 15;143(12):2077-88. doi: 10.1242/dev.130492. Epub 2016 Apr 27. Development. 2016. PMID: 27122176 Free PMC article. - Genetic analyses in Lake Malawi cichlids identify new roles for Fgf signaling in scale shape variation.
Albertson RC, Kawasaki KC, Tetrault ER, Powder KE. Albertson RC, et al. Commun Biol. 2018 May 31;1:55. doi: 10.1038/s42003-018-0060-4. eCollection 2018. Commun Biol. 2018. PMID: 30271938 Free PMC article. - Interspecific differences and ecological correlations between scale number and skin structure in freshwater fishes.
Gu H, Wang H, Zhu S, Yuan D, Dai X, Wang Z. Gu H, et al. Curr Zool. 2022 Aug 10;69(4):491-500. doi: 10.1093/cz/zoac059. eCollection 2023 Aug. Curr Zool. 2022. PMID: 37614923 Free PMC article. - Dorsal spine evolution in threespine sticklebacks via a splicing change in MSX2A.
Howes TR, Summers BR, Kingsley DM. Howes TR, et al. BMC Biol. 2017 Dec 7;15(1):115. doi: 10.1186/s12915-017-0456-5. BMC Biol. 2017. PMID: 29212540 Free PMC article. - Genome sequences reveal global dispersal routes and suggest convergent genetic adaptations in seahorse evolution.
Li C, Olave M, Hou Y, Qin G, Schneider RF, Gao Z, Tu X, Wang X, Qi F, Nater A, Kautt AF, Wan S, Zhang Y, Liu Y, Zhang H, Zhang B, Zhang H, Qu M, Liu S, Chen Z, Zhong J, Zhang H, Meng L, Wang K, Yin J, Huang L, Venkatesh B, Meyer A, Lu X, Lin Q. Li C, et al. Nat Commun. 2021 Feb 17;12(1):1094. doi: 10.1038/s41467-021-21379-x. Nat Commun. 2021. PMID: 33597547 Free PMC article.
References
- Richardson MK. Vertebrate evolution: the developmental origins of adult variation. Bioessays. 1999;21:604–613. - PubMed
- Stern DL. Evolutionary developmental biology and the problem of variation. Evolution Int J Org Evolution. 2000;54:1079–1091. - PubMed
- Sire JY, Huysseune A. Formation of dermal skeletal and dental tissues in fish: a comparative and evolutionary approach. Biological Reviews. 2003;78:219–249. - PubMed
- Huysseune A, Sire JY. Evolution of patterns and processes in teeth and tooth-related tissues in non-mammalian vertebrates. Eur J Oral Sci. 1998;106(Suppl 1):437–481. - PubMed
- Coulombre AJ, Coulombre JL. The skeleton of the eye. I. Conjunctival papillae and scleral ossicles. Dev Biol. 1962;5:382–401. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases