Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity - PubMed (original) (raw)
Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity
Phimon Atsawasuwan et al. J Biol Chem. 2008.
Abstract
Lysyl oxidase (LOX), an amine oxidase critical for the initiation of collagen and elastin cross-linking, has recently been shown to regulate cellular activities possibly by modulating the functions of growth factors. In this study, we investigated the interaction between LOX and transforming growth factor-beta1 (TGF-beta1), a potent growth factor abundant in bone, the effect of LOX on TGF-beta1 signaling, and its potential mechanism. The specific binding between mature LOX and mature TGF-beta1 was demonstrated by immunoprecipitation and glutathione S-transferase pulldown assay in vitro. Both proteins were colocalized in the extracellular matrix in an osteoblastic cell culture system, and the binding complex was identified in the mineral-associated fraction of bone matrix. Furthermore, LOX suppressed TGF-beta1-induced Smad3 phosphorylation likely through its amine oxidase activity. The data indicate that LOX binds to mature TGF-beta1 and enzymatically regulates its signaling in bone and thus may play an important role in bone maintenance and remodeling.
Figures
FIGURE 1.
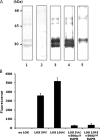
Binding of LOX to TGF-β1 by IP-WB analysis. A, binding of mLOX to TGF-β1 and BMPs. To identify the binding, LOX-HA and TGF-β1-V5 or BMP-2, -4, -6, or -7-V5 (B2, B4, B6, and_B7_) were co-expressed and immunoprecipitated with anti-(α) V5 antibody, and the binding was detected by WB analysis with α HA antibody. The binding of mLOX to TGF-β1(upper panel, lane 6, LOX-HA is indicated by an arrow) but not to any BMPs (upper panel, lanes 2–5) was clearly observed. The expression levels of mLOX-HA and BMPs/TGF-β1-V5 were verified by IP-WB analyses with α HA antibody (middle panel, mLOX-HA is detected at 32 kDa) and with α V5 antibody (lower panel), respectively. An asterisk indicates IgG light chain. B, binding of mLOX to TGF-β1 and its propeptide LAP. The binding was detected by using mLOX-HA and TGF-β1-V5 or LAP-V5. Dose-dependent binding of mLOX-HA to a mixture of full-length (∼62 kDa indicated by an open triangle) and mature TGF-β1-V5 (∼17 kDa indicated by a closed triangle) was observed (upper panel, lanes 3–5, LOX-HA is indicated by an arrow) but not LAP with any doses tested (upper panel, lanes 7–9). The expression levels of mLOX-HA, TGF-β1-V5, and LAP-V5 are shown in the_middle_ and lower panels, respectively. An asterisk indicates IgG light chain. Molecular masses are shown on the_right_.
FIGURE 2.
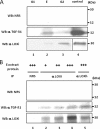
LOX constructs and their binding to TGF-β1 by IP-WB analysis. Four LOX-HA constructs generated, i.e. LOX-HA (A), mature LOX-HA (B), LOX with double mutations (LOXdm-HA) (C), and LOX propeptide (LOXPP-HA) (D), are shown on the left. The binding of each construct to TGF-β1-V5 was analyzed by IP with anti-(α) V5 antibody followed by WB with α HA antibody and shown on right. Expression levels of TGF-β1-V5 are shown by WB with α V5 antibody at the bottom panel on the right. When LOX-HA was expressed, both full-length (50 kDa) and mature LOX-HA (33 kDa) were synthesized (indicated by arrowheads). Note that full-length, mature, and dmLOX-HA showed binding to TGF-β1-V5 in a dose-dependent manner (a–c, lanes 1–3), but no binding was observed for LOXPP-HA (d). The presence of LOXPP-HA (∼28 kDa) in the medium was confirmed by IP-WB analysis with α HA antibody in comparison with the negative control (EV) and is shown in a small inset left to d. LOXPP-HA is indicated by a bracket. IgG heavy (50 kDa) and light (25 kDa) chains are indicated by two and one asterisks, respectively.
FIGURE 3.
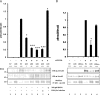
Characterization of recombinant LOX-V5 protein. A, SDS-PAGE and WB analysis. Purified LOX-V5 protein was subjected to SDS-PAGE analysis and stained with Coomassie Brilliant Blue (lane 1) and WB analysis with NRS (lane 2), anti-(α) V5 antibody (lane 3), α LOXi antibody (Imgenex) (lane 4), and α LOXh antibody that was previously reported (27, 28) (lane 5). Two major Coomassie Brilliant Blue-stained bands were observed at ∼30- and ∼35-kDa bands. Both were immunoreactive to α V5 (lane 3) and α LOXi (lane 4) antibodies and the 35 kDa to α LOXh antibody (lane 5). Both proteins were identified as LOX by MALDI-MS (see text). The minor Coomassie Brilliant Blue-stained bands at higher molecular weight region (∼48 and ∼50 kDa) also showed immunoreactivities to those antibodies in a similar manner. No immunoreactivity was found with NRS. B, amine oxidase activity of LOX-V5 protein. Note that the amine oxidase activity of LOX-V5 protein was retained and increased in a dose-dependent manner, and the activity was nullified by 500 μ
m
BAPN. Error bars indicate mean ± S.D. of three independent experiments.
FIGURE 4.
Direct binding of LOX-V5 to mature TGF-β1. A, GST pulldown assay. Ten μg of recombinant LOX-V5 protein (see Fig. 3) was incubated with 2.5 or 5 μg of GST-TGF-β1 or 5 μg of GST and subjected to GST pulldown assay, and the binding was detected by WB analysis with anti-(α) V5 antibody (upper panel). LOX-V5 without pulldown was used as positive control (upper panel, lane 1). No binding was detected to GST alone (lane 2). The binding of GST-TGF-β1 to LOX-V5 was clearly detected in a dose-dependent manner (lanes 3 and 4). WB analysis with α GST for GST and GST-TGF-β1 used (input) are shown in the lower panel. B, direct binding between LOX-V5 and rhTGF-β1 by IP-WB. Twenty ng of rhTGF-β1 was incubated with and without 10 μg of LOX-V5, immunoprecipitated with α V5 antibody, and detected with α TGF-β1 antibody (upper panel). An immunoreactive band was detected when LOX-V5 was incubated with rhTGF-β1(upper panel, lane 2) but absent when LOX-V5 protein was not added (upper panel, lane 1). WB analysis with α TGF-β1 for rhTGF-β1 used (input) is shown in the lower panel.
FIGURE 5.
Co-localization of LOX and TGF-β1 in an MC cell culture system. After 3 weeks of culture, immunofluorescence staining for LOX and TGF-β1 was performed and observed under laser-scanning confocal microscopy.A, TGF-β1 is shown in red; B, LOX is shown in_green_; D, merged image of A and B showing colocalization of the two molecules in yellow. An enlarged image (inset) of the merged ECM is shown on the right. C, differential interference contrast (DIC) image confirming the fibrous ECM (indicated by an arrow). An arrowhead indicates a nucleus.
FIGURE 6.
Binding of LOX and TGF-β1 in bone matrix. A, presence of LOX and TGF-β1 proteins in bone matrix extracts. WB analyses were performed with anti-(α) LOXi antibody (lower panel), α TGF-β1 antibody (middle panel), and NRS (upper panel). The immunoreactive bands for LOX and TGF-β1 were detected at the expected molecular weight in E and G2 fraction of bone (lanes 2 and_3_) but not in G1 fraction (lane 1). No immunoreactive bands were detected with NRS (upper panel). G, guanidine-HCl;G1, first G extract; E, EDTA extract; G2, second G extract. LOX isolated from bovine aorta by the method reported (75) and rhTGF-β1 were used as positive controls (lane 4). B, LOX-TGF-β1 binding complex in bone E extract. Various amounts of E extract were subjected to IP-WB analysis in combination of α LOXi, α LOXh, α TGF-β1 antibody, or NRS as indicated. Note that immunopositive bands of TGF-β1 are detected in a dose-dependent manner (middle panel, lanes 2–4) when IP was performed with α LOXi. An immunopositive band was also observed when IP was performed with α LOXh antibody (lane 5). No immunoreactivity was detected when NRS was used (upper panel, lanes 1–5, middle/lower panel, lane 1). +, ++, +++, 500, 1000, and 2000 μg, respectively, of E extract protein.
FIGURE 7.
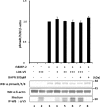
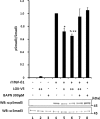
Effect of LOX as an amine oxidase on TGF-β signaling in MC cells. A, effect of LOX overexpression and its amine oxidase activity on TGF-β signaling. TGF-β1 signaling was measured as phospho(p)-Smad3 relative to total Smad3 (pSmad3/Smad3). Without the treatment with rhTGF-β1, Smad3 phosphorylation was not induced in MC cells transfected with EV (lane 1) or those overexpressing LOX-V5 (lane 2). When rhTGF-β1(5 ng/ml) was added to EV for 30 min, Smad3 phosphorylation was induced (lane 3); however, the rhTGF-β1-induced phosphorylation level was decreased when LOX-V5 was expressed in a dose-dependent manner (lanes 4–6). The LOX inhibition of TGF-β1-induced Smad3 phosphorylation was not affected in the presence of 200 units/ml catalase (lane 7) but completely rescued in the presence of 300 μ
m
BAPN (lane 8). Error bars indicate mean ± S.D. of three independent experiments. *, significantly different (p < 0.05) from EV. * and **, significantly different (p < 0.05) from EV and LOX+. B, effects of enzymatically active and inactive LOX on TGF-β1-induced Smad3 phosphorylation. Without TGF-β1, no phosphorylation was observed in EV (lane 1), those expressing LOX-V5 (lane 2), or LOXdm-V5 (lane 3). Smad3 phosphorylation was induced upon the treatment with rhTGF-β1 (5 ng/ml) for 30 min in EV (lane 4); however, it was significantly down-regulated by active LOX-V5 (lane 5) but almost unaffected by inactive LOXdm (lane 6). The level of LOX expression was determined by IP-WB withα LOXi antibody (lower panel). Error bars indicate mean ± S.D. of three independent experiments. *, significantly different (p < 0.05) from EV. +, ++, and +++ indicate the relative amount of EV or LOX expression vectors transfected.
FIGURE 8.
Effect of LOX overexpression on BMP-2 signaling in MC cells. The BMP-2-induced signaling was calculated as phospho-Smad1/5/8 relative to β-actin. Without rhBMP-2 treatment, Smad 1/5/8 phosphorylation was not induced in cells transfected by EV (lane 1) and those overexpressing LOX-V5 (lane 2). Upon rhBMP-2 treatment (100 ng/ml) for 30 min, the phosphorylation was induced in EV (lane 3). The level of BMP-2-induced Smad1/5/8 phosphorylation was not affected with the presence of various levels of LOX-V5 (lanes 4–6). The presence of BAPN did not affect the phosphorylation levels (lanes 7 and 8). The level of LOX-V5 protein was determined by IP-WB with α V5 antibody (lower panel). +, ++, and +++ indicate the relative amount of EV or LOX expression vectors transfected.
FIGURE 9.
Effect of exogenous addition of LOX-V5 on TGF-β signaling in MC cells. Without rhTGF-β1, Smad3 phosphorylation was not induced in MC cells (lane 1) and those treated with LOX-V5 (lane 2) or BAPN (lane 3). The phosphorylation was induced in EV upon 5 ng/ml rhTGF-β1 treatment for 30 min (lane 4), but the phosphorylation level (calculated as pSmad3/Smad3) decreased with the addition of LOX-V5 in a dose-dependent manner (lanes 5 and 6). The inhibition of TGF-β1-induced Smad3 phosphorylation by LOX was rescued by the presence of 300 μ
m
BAPN (lane 7). With the presence of BAPN (lane 8), rhTGF-β1-induced Smad3 phosphorylation was comparable with that of control (lane 4). Error bars indicate mean ± S.D. of three independent experiments. *, significantly different (p < 0.05) from lane 4.* and **, significantly different (p < 0.05) from lanes 4 and 5.
FIGURE 10.
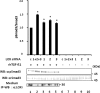
Effect of LOX suppression by RNA interference on TGF-β signaling in MC cells. When MC cells were transfected with silencer negative control (c) and treated with TGF-β1, Smad3 phosphorylation was induced (lane 1). However, the Smad3 phosphorylation level (pSmad3/Smad3) was further enhanced when LOX expression was suppressed when RNA interference was performed (lanes 2–5). The increase in signaling was enhanced the most in the cells transfected with three siRNA constructs combined (1 + 2 + 3, lane 2) that expressed the lowest level of LOX when compared with those transfected with individual constructs (1, lane 3;2, lane 4, and 3, lane 5). Without TGF-β induction, the phosphorylation was not detected in any of those cell groups (lanes 6–10). The level of LOX expression was determined by IP-WB with α LOXi antibody (bottom panel).Error bars indicate mean ± S.D. of three independent experiments. *, significantly different (p < 0.05) from control (lane 1).
Similar articles
- Regulation of elastin promoter by lysyl oxidase and growth factors: cross control of lysyl oxidase on TGF-beta1 effects.
Oleggini R, Gastaldo N, Di Donato A. Oleggini R, et al. Matrix Biol. 2007 Jul;26(6):494-505. doi: 10.1016/j.matbio.2007.02.003. Epub 2007 Feb 27. Matrix Biol. 2007. PMID: 17395448 - Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells.
Taylor MA, Amin JD, Kirschmann DA, Schiemann WP. Taylor MA, et al. Neoplasia. 2011 May;13(5):406-18. doi: 10.1593/neo.101086. Neoplasia. 2011. PMID: 21532881 Free PMC article. - Downregulation of lysyl oxidase and lysyl oxidase-like protein 2 suppressed the migration and invasion of trophoblasts by activating the TGF-β/collagen pathway in preeclampsia.
Xu XH, Jia Y, Zhou X, Xie D, Huang X, Jia L, Zhou Q, Zheng Q, Zhou X, Wang K, Jin LP. Xu XH, et al. Exp Mol Med. 2019 Feb 21;51(2):1-12. doi: 10.1038/s12276-019-0211-9. Exp Mol Med. 2019. PMID: 30804321 Free PMC article. - Lysyl Oxidase (LOX): Functional Contributions to Signaling Pathways.
Laczko R, Csiszar K. Laczko R, et al. Biomolecules. 2020 Jul 22;10(8):1093. doi: 10.3390/biom10081093. Biomolecules. 2020. PMID: 32708046 Free PMC article. Review. - Lysyl oxidases: a novel multifunctional amine oxidase family.
Csiszar K. Csiszar K. Prog Nucleic Acid Res Mol Biol. 2001;70:1-32. doi: 10.1016/s0079-6603(01)70012-8. Prog Nucleic Acid Res Mol Biol. 2001. PMID: 11642359 Review.
Cited by
- Effect of phenytoin and age on gingival fibroblast enzymes.
Vahabi S, Nazemisalman B, Vahid Golpaigani M, Ahmadi A. Vahabi S, et al. J Dent (Tehran). 2014 May;11(3):270-81. Epub 2014 May 31. J Dent (Tehran). 2014. PMID: 25628662 Free PMC article. - Molecular mechanisms of thoracic aortic dissection.
Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Wu D, et al. J Surg Res. 2013 Oct;184(2):907-24. doi: 10.1016/j.jss.2013.06.007. Epub 2013 Jun 29. J Surg Res. 2013. PMID: 23856125 Free PMC article. Review. - Mechanotransduction at focal adhesions: from physiology to cancer development.
Seong J, Wang N, Wang Y. Seong J, et al. J Cell Mol Med. 2013 May;17(5):597-604. doi: 10.1111/jcmm.12045. Epub 2013 Apr 18. J Cell Mol Med. 2013. PMID: 23601032 Free PMC article. Review. - Lessons on the pathogenesis of aneurysm from heritable conditions.
Lindsay ME, Dietz HC. Lindsay ME, et al. Nature. 2011 May 19;473(7347):308-16. doi: 10.1038/nature10145. Nature. 2011. PMID: 21593863 Free PMC article. Review. - Human colorectal cancer progression correlates with LOX-induced ECM stiffening.
Wei B, Zhou X, Liang C, Zheng X, Lei P, Fang J, Han X, Wang L, Qi C, Wei H. Wei B, et al. Int J Biol Sci. 2017 Nov 1;13(11):1450-1457. doi: 10.7150/ijbs.21230. eCollection 2017. Int J Biol Sci. 2017. PMID: 29209148 Free PMC article.
References
- Kagan, H. M., and Li, W. (2003) J. Cell Biochem. 88 660-672 - PubMed
- Yeager, V. L., Buranarugsa, M. W., and Arunatut, O. (1985) J. Exp. Pathol. 2 1-11 - PubMed
- Ekholm, E. C., Ravanti, L., Kahari, V., Paavolainen, P., and Penttinen, R. P. (2000) Bone (Elmsford) 27 551-557 - PubMed
- Li, W., Nugent, M. A., Zhao, Y., Chau, A. N., Li, S. J., Chou, I. N., Liu, G., and Kagan, H. M. (2003) J. Cell Biochem. 88 152-164 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases