CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease - PubMed (original) (raw)
. 2008 Nov;9(11):1253-60.
doi: 10.1038/ni.1658. Epub 2008 Oct 5.
Affiliations
- PMID: 18836452
- PMCID: PMC2901237
- DOI: 10.1038/ni.1658
CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease
Husein Hadeiba et al. Nat Immunol. 2008 Nov.
Abstract
Dendritic cells (DCs) are 'professional' antigen-presenting cells that are key in the regulation of immune responses. Here we characterize a unique subset of tolerogenic DCs that expressed the chemokine receptor CCR9 and migrated to the CCR9 ligand CCL25, a chemokine linked to the homing of T cells and DCs to the gut. CCR9(+) DCs were of the plasmacytoid DC (pDC) lineage, had an immature phenotype and rapidly downregulated CCR9 in response to maturation-inducing pDC-restricted Toll-like receptor ligands. CCR9(+) pDCs were potent inducers of regulatory T cell function and suppressed antigen-specific immune responses both in vitro and in vivo, including inhibiting acute graft-versus-host disease induced by allogeneic CD4(+) donor T cells in irradiated recipients. Our results identify a highly immunosuppressive population of pDCs present in lymphoid tissues.
Figures
Figure 1. Tissue-specific CCR9 expression profiles of DCs
CD11c+ DCs were analyzed for surface expression of CCR9 and MHC class II in mesenteric lymph nodes (MLN), peripheral lymph nodes (PLN), spleen (SPLN) and blood of normal BALB/c mice. Cells were gated on CD11c+ DCs. One of two representative experiments is shown.
Figure 2. CCR9+ DCs reside in the plasmacytoid DC compartment and have a predominantly immature phenotype
(a) DCs from pooled peripheral lymph nodes (PLN) were isolated from normal C57BL/6 mice. Cell suspensions were stained and gated on plasmacytoid (CD11cint B220+) and non-plasmacytoid (CD11chi B220−) lineage− DCs (pDCs and non-pDCs respectively). The gated cells were analyzed for expression of MHC class II and costimulatory markers CD80, CD86 and CD40 to assess their maturation state and correlated with CCR9 expression. Gates were set based on isotype controls for MHC class II and costimulatory markers. Representative FACS plots from one out of three mice. (b) Within the gated pDC and non-pDC subsets, the percentage of CCR9+ cells of gated DC subsets was assessed by FACS analysis in blood and various lymphoid tissues indicated (PLN, peripheral lymph nodes; MLN, mesenteric lymph nodes). Error bars represent the SEM from three mice. (c) DCs from pooled PLN expanded by Flt3L-secreting B16 melanoma cells in C57BL/6 mice, were gated on pDCs (CD11cint B220+) and non-pDCs (CD11chi B220−) lineage− DCs. The gated cells were analyzed for expression of MHC class II and costimulatory markers CD80 and CD40 to assess their maturation state and correlated with CCR9 expression. Gates were set based on isotype controls for MHC class II, CD80 and CD40. Representative FACS plots from one out of three mice.
Figure 3. CCR9 expression allows pDC migration to CCL25
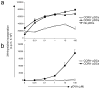
Chemotactic response of DC subsets from pooled lymph node cells of Flt3L-treated B6 mice toward the CXCR4 ligand CXCL12, CCR7 ligand CCL21, and the CCR9 ligand CCL25. DC subsets were subdivided into pDCs and non-pDCs as described (a) and pDCs were subdivided based on their expression of CCR9 (b). Results are expressed as the mean percentage of total cells migrating towards the chemokine or towards the medium alone. Error bars represent the SEM of triplicate wells with representative results from one out of two experiments. *, P = 0.001, **, P < 0.001 by _t_-test comparing migration of pDCs versus non-pDCs to CXCL12 or CCL25, respectively. Similarly #, P = 0.008, ##, P < 0.001 comparing migration of CCR9+ versus CCR9− pDCs to CCL21 or CCL25, respectively.
Figure 4. CCR9+ DCs downregulate CCR9 after activation with pDC-specific TLR ligands
(a) Sorted CCR9+ pDCs from pooled PLNs isolated from Flt3L-treated B6 mice were left untreated or activated in the presence of LPS (1 ng/ml), CpG (1 μM) and R-837 (10 μg/ml) for 8–12 h. Cells were stained for CCR9, MHC class II (I-Ab) and the costimulatory ligands CD80 and CD40. Gates were based on isotype controls with control antibody staining yielding <1% of cells in the positive gates. Representative FACS plots shown from one of three experiments with similar results. (b) Sorted CCR9+ and CCR9− pDCs were also activated in the presence of LPS (10 ng/ml) or escalating doses of CpG (2 and 20 μg/ml) overnight for 16 h and supernatants were assayed for IFN-α and TNF–α. Results are expressed as mean concentration (pg/ml) of duplicate cultures. Data presented are from one of two experiments for IFN-α and one of three experiments for TNF-α with similar results.
Figure 4. CCR9+ DCs downregulate CCR9 after activation with pDC-specific TLR ligands
(a) Sorted CCR9+ pDCs from pooled PLNs isolated from Flt3L-treated B6 mice were left untreated or activated in the presence of LPS (1 ng/ml), CpG (1 μM) and R-837 (10 μg/ml) for 8–12 h. Cells were stained for CCR9, MHC class II (I-Ab) and the costimulatory ligands CD80 and CD40. Gates were based on isotype controls with control antibody staining yielding <1% of cells in the positive gates. Representative FACS plots shown from one of three experiments with similar results. (b) Sorted CCR9+ and CCR9− pDCs were also activated in the presence of LPS (10 ng/ml) or escalating doses of CpG (2 and 20 μg/ml) overnight for 16 h and supernatants were assayed for IFN-α and TNF–α. Results are expressed as mean concentration (pg/ml) of duplicate cultures. Data presented are from one of two experiments for IFN-α and one of three experiments for TNF-α with similar results.
Figure 5. CCR9+ DCs suppress immune responses in vivo and in vitro
(a) Sorted CCR9+ or CCR9− pDCs from pooled PLNs isolated from Flt3L-treated B6 mice were pulsed for 2–4 h with OVA peptide prior to i.v. administration of naive C57BL/6 mice. Control mice did not receive pDCs. Recipient mice were boosted 1 week later with the same Ag-loaded pDCs and immunized s.c. one week after the final boost with pOVA emulsified in CFA. After 10 days, cell suspensions from draining lymph nodes were stimulated with a dose range of pOVA for 72 h prior to the addition of 3H-thymidine and subsequent determination of cellular proliferation. Results are expressed as mean c.p.m. and SEM (shown as error bars) of quadruplicate cultures. Data presented are from one of two experiments with similar results. (b) CD4+ T cells MACS-purified from spleens of BALB/c mice were cultured with sorted CCR9+ or CCR9− pDCs from pooled lymph nodes of Flt3L-treated B6 mice at a 5:1 ratio. T cell proliferation to a dose range of pOVA was determined 72 h later by the incorporation of 3H-thymidine for an additional 18 h. Results are expressed as mean cpm of triplicate cultures with error bars denoting the SEM and represent one out of two experiments.
Figure 6. CCR9+ pDCs are potent inducers of regulatory T cells in vitro
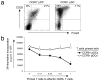
(a) Splenic CD4+ BALB/c T cells (106) were cultured for 5 days with sorted CCR9+ or CCR9− pDCs (0.2 × 106 cells) from pooled lymph nodes isolated from Flt3L-treated B6 mice. T cell expression of intracellular Foxp3 and cell surface CD25 was determined by flow cytometry. Cells were gated on CD4+ lymphocytes, and the gates were set based on the isotype controls shown for the anti-Foxp3 and anti-CD25 mAbs. One of 2 representative experiments is shown. (b) CD4+ T cells cultured in vitro after 5 days with either CCR9+ pDCs or CCR9− pDCs were added in increasing numbers to 105 freshly isolated BALB/c CD4+ CD25− effector T cells in anti-CD3 and anti-CD28 coated wells. After 48 h, cultures were pulsed with 1 μCi of 3H-thymidine for an additional 18 h, and then harvested. Error bars represent the SEM of triplicate cultures.
Figure 7. Lethal GVHD of C57BL/6 recipients induced by BALB/c CD4+ CD25− effector T cells can be suppressed by co-injected C57BL/6 CCR9+ DCs
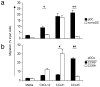
(a) C57BL/6 mice received 2 × 450 rads of total body irradiation, 2 × 106 BALB/c T-cell depleted bone marrow cells and 0.5–1 × 106 BALB/c splenic CD4+CD25− T cells. Three cohorts of mice received either coinjected sorted CCR9+ pDCs, CCR9+ pDCs or no pDCs at all (no DC control) at 0.2–0.5 × 106 DCs/mouse from pooled peripheral lymph nodes of Flt3L-treated B6 mice. Combined data from two independent experiments are shown, with a total of 9–10 animals per treatment group. The two independent experiments showed similar results with roughly 50% mortality in the absence of protection by CCR9+ pDCs. *, P = 0.027, **, P = 0.010 using the logrank test comparing the survival of allogeneic bone marrow and effector T cell treated mice receiving CCR9+ pDCs versus no pDCs or CCR9+ pDCs versus CCR9− pDCs respectively. (b) Intracellular cytokine staining of splenocytes and peripheral lymph nodes from irradiated C57BL/6 (Thy1.2+) mice three weeks after i.v. transfer of 2 × 106 BALB/c (Thy1.2+) T-cell depleted bone marrow cells, 0.5–1 × 106 splenic Thy1.1+ effector CD4+CD25− T cells from congenic BALB/c.Thy1.1 mice and either no pDCs or sorted CCR9+ or CCR9− pDCs from Flt3L-treated B6 (Thy1.2+) mice. Cells were gated on CD4+ Thy1.1+ effector T cells and each bar represents the percentage of gated cells producing intracellular IL-17 and IFN-γ from 2–3 pooled mice. Data presented are from one of two experiments with similar results. (c) Analysis of the same groups of mice in B for the expression of intracellular foxp3 and surface CD25 from unstimulated splenocytes and MLN cells. Cells were gated on CD4+ Thy1.1+ effector T cells and the gates were set based on the isotype controls for the anti-Foxp3 and anti-CD25 mAbs to include <1% in the positive stained gates. Representative FACS plots from two experiments.
Figure 7. Lethal GVHD of C57BL/6 recipients induced by BALB/c CD4+ CD25− effector T cells can be suppressed by co-injected C57BL/6 CCR9+ DCs
(a) C57BL/6 mice received 2 × 450 rads of total body irradiation, 2 × 106 BALB/c T-cell depleted bone marrow cells and 0.5–1 × 106 BALB/c splenic CD4+CD25− T cells. Three cohorts of mice received either coinjected sorted CCR9+ pDCs, CCR9+ pDCs or no pDCs at all (no DC control) at 0.2–0.5 × 106 DCs/mouse from pooled peripheral lymph nodes of Flt3L-treated B6 mice. Combined data from two independent experiments are shown, with a total of 9–10 animals per treatment group. The two independent experiments showed similar results with roughly 50% mortality in the absence of protection by CCR9+ pDCs. *, P = 0.027, **, P = 0.010 using the logrank test comparing the survival of allogeneic bone marrow and effector T cell treated mice receiving CCR9+ pDCs versus no pDCs or CCR9+ pDCs versus CCR9− pDCs respectively. (b) Intracellular cytokine staining of splenocytes and peripheral lymph nodes from irradiated C57BL/6 (Thy1.2+) mice three weeks after i.v. transfer of 2 × 106 BALB/c (Thy1.2+) T-cell depleted bone marrow cells, 0.5–1 × 106 splenic Thy1.1+ effector CD4+CD25− T cells from congenic BALB/c.Thy1.1 mice and either no pDCs or sorted CCR9+ or CCR9− pDCs from Flt3L-treated B6 (Thy1.2+) mice. Cells were gated on CD4+ Thy1.1+ effector T cells and each bar represents the percentage of gated cells producing intracellular IL-17 and IFN-γ from 2–3 pooled mice. Data presented are from one of two experiments with similar results. (c) Analysis of the same groups of mice in B for the expression of intracellular foxp3 and surface CD25 from unstimulated splenocytes and MLN cells. Cells were gated on CD4+ Thy1.1+ effector T cells and the gates were set based on the isotype controls for the anti-Foxp3 and anti-CD25 mAbs to include <1% in the positive stained gates. Representative FACS plots from two experiments.
Comment in
- Literature watch: implications for transplantation.
Alessandrini A, Bromberg JS. Alessandrini A, et al. Am J Transplant. 2012 Nov;12(11):2867. doi: 10.1111/j.1600-6143.2012.04330.x. Am J Transplant. 2012. PMID: 23107267 No abstract available.
Similar articles
- Impaired CCR9/CCL25 signalling induced by inefficient dendritic cells contributes to intestinal immune imbalance in nonalcoholic steatohepatitis.
Gao W, Wang Y, Bi J, Chen X, Li N, Wang Y, Tang H, Mao J. Gao W, et al. Biochem Biophys Res Commun. 2021 Jan 1;534:34-40. doi: 10.1016/j.bbrc.2020.12.007. Epub 2020 Dec 10. Biochem Biophys Res Commun. 2021. PMID: 33310185 - CCR9 signaling in dendritic cells drives the differentiation of Foxp3+ Tregs and suppresses the allergic IgE response in the gut.
Pathak M, Padghan P, Halder N, Shilpi, Kulkarni N, Sonar SA, Lal G. Pathak M, et al. Eur J Immunol. 2020 Mar;50(3):404-417. doi: 10.1002/eji.201948327. Epub 2019 Dec 1. Eur J Immunol. 2020. PMID: 31755547 - Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance.
Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Hadeiba H, et al. Immunity. 2012 Mar 23;36(3):438-50. doi: 10.1016/j.immuni.2012.01.017. Immunity. 2012. PMID: 22444632 Free PMC article. - The Regulatory Function of CCR9+ Dendritic Cells in Inflammation and Autoimmunity.
Pathak M, Lal G. Pathak M, et al. Front Immunol. 2020 Oct 2;11:536326. doi: 10.3389/fimmu.2020.536326. eCollection 2020. Front Immunol. 2020. PMID: 33123124 Free PMC article. Review. - Regulation of the immune and inflammatory responses by the 'atypical' chemokine receptor D6.
Graham GJ, Locati M. Graham GJ, et al. J Pathol. 2013 Jan;229(2):168-75. doi: 10.1002/path.4123. J Pathol. 2013. PMID: 23125030 Review.
Cited by
- Immunomodulatory Functions of BTLA and HVEM Govern Induction of Extrathymic Regulatory T Cells and Tolerance by Dendritic Cells.
Jones A, Bourque J, Kuehm L, Opejin A, Teague RM, Gross C, Hawiger D. Jones A, et al. Immunity. 2016 Nov 15;45(5):1066-1077. doi: 10.1016/j.immuni.2016.10.008. Epub 2016 Oct 25. Immunity. 2016. PMID: 27793593 Free PMC article. - Food allergy: immune mechanisms, diagnosis and immunotherapy.
Yu W, Freeland DMH, Nadeau KC. Yu W, et al. Nat Rev Immunol. 2016 Dec;16(12):751-765. doi: 10.1038/nri.2016.111. Epub 2016 Oct 31. Nat Rev Immunol. 2016. PMID: 27795547 Free PMC article. Review. - Granzyme B produced by human plasmacytoid dendritic cells suppresses T-cell expansion.
Jahrsdörfer B, Vollmer A, Blackwell SE, Maier J, Sontheimer K, Beyer T, Mandel B, Lunov O, Tron K, Nienhaus GU, Simmet T, Debatin KM, Weiner GJ, Fabricius D. Jahrsdörfer B, et al. Blood. 2010 Feb 11;115(6):1156-65. doi: 10.1182/blood-2009-07-235382. Epub 2009 Dec 3. Blood. 2010. PMID: 19965634 Free PMC article. - CCL25 Signaling in the Tumor Microenvironment.
Mir H, Singh S. Mir H, et al. Adv Exp Med Biol. 2021;1302:99-111. doi: 10.1007/978-3-030-62658-7_8. Adv Exp Med Biol. 2021. PMID: 34286444 - Imiquimod-induced CCR9 Ameliorates murine TNBS Colitis.
Suzuki R, Katakura K, Fujiwara T, Gunji N, Watanabe H, Ohira H. Suzuki R, et al. Fukushima J Med Sci. 2016 Dec 16;62(2):90-100. doi: 10.5387/fms.2015-28. Epub 2016 Nov 10. Fukushima J Med Sci. 2016. PMID: 27829595 Free PMC article.
References
- Banchereau J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. - PubMed
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. - PubMed
- van Duivenvoorde LM, van Mierlo GJ, Boonman ZF, Toes RE. Dendritic cells: vehicles for tolerance induction and prevention of autoimmune diseases. Immunobiology. 2006;211:627–632. - PubMed
- Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. - PubMed
- Charbonnier LM, et al. Immature dendritic cells suppress collagen-induced arthritis by in vivo expansion of CD49b+ regulatory T cells. J Immunol. 2006;177:3806–3813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI007290/AI/NIAID NIH HHS/United States
- R37 GM037734/GM/NIGMS NIH HHS/United States
- R03 DK069395/DK/NIDDK NIH HHS/United States
- K08DK069385/DK/NIDDK NIH HHS/United States
- R01 DK084647/DK/NIDDK NIH HHS/United States
- K08 DK069385/DK/NIDDK NIH HHS/United States
- R03DK069395/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials