Mechanotransduction, asthma, and airway smooth muscle - PubMed (original) (raw)
Mechanotransduction, asthma, and airway smooth muscle
Ben Fabry et al. Drug Discov Today Dis Models. 2007.
Abstract
Excessive force generation by airway smooth muscle is the main culprit in excessive airway narrowing during an asthma attack. The maximum force the airway smooth muscle can generate is exquisitely sensitive to muscle length fluctuations during breathing, and is governed by complex mechanotransduction events that can best be studied by a hybrid approach in which the airway wall is modeled in silico so as to set a dynamic muscle load comparable to that experienced in vivo.
Figures
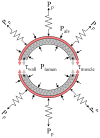
Fig. 1
Schematic cross-section of an airway and surrounding lung parenchyma. The airway is held open by the tethering stress of the lung parenchyma (Pp) and is constricted by the airway smooth muscle tension (τmuscle). Other forces that can either open or close the airway result from the differences between the alveolar pressure (Palv) and airway lumen pressure (Plumen), and the passive tension from stretching or compressing the airway wall tissue (τwall).
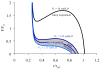
Fig. 2
Steady-state load curve according to Eq. 1 for an 8th generation human airway during quiet breathing and deep inspiration. Here, the lumen and alveolar pressure are assumed to be equilibrated (zero flow condition), hence transpulmonary pressure equals pleura pressure. The load curve is normalized to the maximal force F0 that the smooth muscle of that airway can generate, and to a reference radius rref of the unconstricted airway at a transmural pressure of 10 cmH2O.
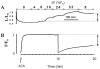
Fig. 3
Smooth muscle response to large-scale force and length perturbations shows non-reversible behavior attributable to cytoskeletal remodeling. A: Muscle length vs. time during stimulation with acetylcholine. The first 120 min correspond to an isotonic shortening at F = 0.32 F0. The muscle lengthens in response to sinusoidal force perturbations with amplitudes between 4% and 32% of maximum force (F0) around a mean force of 0.32 F0. After force perturbations are reduced to 8%, the muscle shortens incompletely (arrow). Adapted from Ref. [5]. B: Force vs. time during an isometric contraction at length L = L0. After 10 min, the muscle length is reduced to 0.7 L0. The subsequent force recovery is incomplete (arrow). Adapted from Ref. [12].
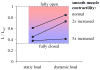
Fig. 4
Length of fully activated smooth muscle in an 8th generation human airway for a static load (constant pleural pressure of 4 cmH2O) and a dynamic load. The dynamic load consisted of quiet breathing (pleural pressure amplitude 1.25 cmH2O, 12 breaths/min), punctuated by deep inspirations (10 cmH2O amplitude every 6 min). Muscle length was measured under the loading conditions for a normal airway, a mildly asthmatic airway with a 2-fold increase in smooth muscle mass, and a severely asthmatic airway with a 5-fold increase in smooth muscle mass. Dynamic loading lead to a substantial bronchodilation for both the normal and the mildly asthmatic airway, but failed to relax the severely asthmatic airway. Adapted from Ref. [38].
Similar articles
- The mechanics of exaggerated airway narrowing in asthma: the role of smooth muscle.
King GG, Paré PD, Seow CY. King GG, et al. Respir Physiol. 1999 Oct 15;118(1):1-13. doi: 10.1016/s0034-5687(99)00076-6. Respir Physiol. 1999. PMID: 10568415 Review. - The role of airway smooth muscle during an attack of asthma simulated in vitro.
McParland BE, Tait RR, Paré PD, Seow CY. McParland BE, et al. Am J Respir Cell Mol Biol. 2005 Nov;33(5):500-4. doi: 10.1165/rcmb.2005-0183OC. Epub 2005 Jul 29. Am J Respir Cell Mol Biol. 2005. PMID: 16055669 - Dynamic equilibration of airway smooth muscle contraction during physiological loading.
Latourelle J, Fabry B, Fredberg JJ. Latourelle J, et al. J Appl Physiol (1985). 2002 Feb;92(2):771-9. doi: 10.1152/japplphysiol.01090.2000. J Appl Physiol (1985). 2002. PMID: 11796691 - The functional consequences of airway remodeling in asthma.
Paré PD, Roberts CR, Bai TR, Wiggs BJ. Paré PD, et al. Monaldi Arch Chest Dis. 1997 Dec;52(6):589-96. Monaldi Arch Chest Dis. 1997. PMID: 9550873 Review. - Airway remodeling: potential contributions of subepithelial fibrosis and airway smooth muscle hypertrophy/hyperplasia to airway narrowing in asthma.
Bento AM, Hershenson MB. Bento AM, et al. Allergy Asthma Proc. 1998 Nov-Dec;19(6):353-8. doi: 10.2500/108854198778612672. Allergy Asthma Proc. 1998. PMID: 9876774 Review.
Cited by
- Physically-induced cytoskeleton remodeling of cells in three-dimensional culture.
Lee SL, Nekouzadeh A, Butler B, Pryse KM, McConnaughey WB, Nathan AC, Legant WR, Schaefer PM, Pless RB, Elson EL, Genin GM. Lee SL, et al. PLoS One. 2012;7(12):e45512. doi: 10.1371/journal.pone.0045512. Epub 2012 Dec 27. PLoS One. 2012. PMID: 23300512 Free PMC article. - Single-cell response to stiffness exhibits muscle-like behavior.
Mitrossilis D, Fouchard J, Guiroy A, Desprat N, Rodriguez N, Fabry B, Asnacios A. Mitrossilis D, et al. Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18243-8. doi: 10.1073/pnas.0903994106. Epub 2009 Oct 5. Proc Natl Acad Sci U S A. 2009. PMID: 19805036 Free PMC article. - Gα/GSA-1 works upstream of PKA/KIN-1 to regulate calcium signaling and contractility in the Caenorhabditis elegans spermatheca.
Castaneda PG, Cecchetelli AD, Pettit HN, Cram EJ. Castaneda PG, et al. PLoS Genet. 2020 Aug 10;16(8):e1008644. doi: 10.1371/journal.pgen.1008644. eCollection 2020 Aug. PLoS Genet. 2020. PMID: 32776941 Free PMC article. - Effect of aging on cellular mechanotransduction.
Wu M, Fannin J, Rice KM, Wang B, Blough ER. Wu M, et al. Ageing Res Rev. 2011 Jan;10(1):1-15. doi: 10.1016/j.arr.2009.11.002. Epub 2009 Nov 20. Ageing Res Rev. 2011. PMID: 19932197 Free PMC article. Review. - Emergence of airway smooth muscle functions related to structural malleability.
Seow CY, Fredberg JJ. Seow CY, et al. J Appl Physiol (1985). 2011 Apr;110(4):1130-5. doi: 10.1152/japplphysiol.01192.2010. Epub 2010 Dec 2. J Appl Physiol (1985). 2011. PMID: 21127211 Free PMC article. Review.
References
- Gunst SJ, et al. Mechanisms for the mechanical plasticity of tracheal smooth muscle. Am J Physiol. 1995;268(5 Pt 1):C1267–1276. - PubMed
- Fredberg JJ, et al. Friction in airway smooth muscle: mechanism, latch, and implications in asthma. J Appl Physiol. 1996;81(6):2703–2712. - PubMed
- Fredberg JJ, et al. Airway smooth muscle, tidal stretches, and dynamically determined contractile states. Am J Respir Crit Care Med. 1997;156(6):1752–1759. - PubMed
- Raboudi SH, et al. Dynamically determined contractile states of airway smooth muscle. Am J Respir Crit Care Med. 1998;158(5 Pt 3):S176–178. - PubMed
- Fredberg JJ, et al. Perturbed equilibrium of myosin binding in airway smooth muscle and its implications in bronchospasm. Am J Respir Crit Care Med. 1999;159(3):959–967. - PubMed
Grants and funding
- R01 HL059682-09/HL/NHLBI NIH HHS/United States
- P01 HL033009-200004/HL/NHLBI NIH HHS/United States
- R01 HL059682/HL/NHLBI NIH HHS/United States
- R01 HL102373/HL/NHLBI NIH HHS/United States
- P01 HL033009/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources