AKT alters genome-wide estrogen receptor alpha binding and impacts estrogen signaling in breast cancer - PubMed (original) (raw)
AKT alters genome-wide estrogen receptor alpha binding and impacts estrogen signaling in breast cancer
Poornima Bhat-Nakshatri et al. Mol Cell Biol. 2008 Dec.
Abstract
Estrogen regulates several biological processes through estrogen receptor alpha (ERalpha) and ERbeta. ERalpha-estrogen signaling is additionally controlled by extracellular signal activated kinases such as AKT. In this study, we analyzed the effect of AKT on genome-wide ERalpha binding in MCF-7 breast cancer cells. Parental and AKT-overexpressing cells displayed 4,349 and 4,359 ERalpha binding sites, respectively, with approximately 60% overlap. In both cell types, approximately 40% of estrogen-regulated genes associate with ERalpha binding sites; a similar percentage of estrogen-regulated genes are differentially expressed in two cell types. Based on pathway analysis, these differentially estrogen-regulated genes are linked to transforming growth factor beta (TGF-beta), NF-kappaB, and E2F pathways. Consistent with this, the two cell types responded differently to TGF-beta treatment: parental cells, but not AKT-overexpressing cells, required estrogen to overcome growth inhibition. Combining the ERalpha DNA-binding pattern with gene expression data from primary tumors revealed specific effects of AKT on ERalpha binding and estrogen-regulated expression of genes that define prognostic subgroups and tamoxifen sensitivity of ERalpha-positive breast cancer. These results suggest a unique role of AKT in modulating estrogen signaling in ERalpha-positive breast cancers and highlights how extracellular signal activated kinases can change the landscape of transcription factor binding to the genome.
Figures
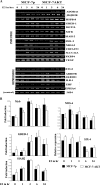
FIG. 1.
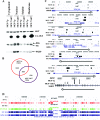
AKT affects ERα binding to the genome. (A) Subcellular distribution pattern of CA-AKT and its effect on ERα levels. Nuclear, cytoplasmic, soluble, and membrane fractions of MCF-7p (control) and MCF-7AKT cells were subjected to Western analysis for AKT and ERα. Blots were reprobed for PARP to ensure the enrichment of nuclear proteins in the nuclear fraction. (B) Summary of ERα DNA-binding pattern to the genome of MCF-7p and MCF-7AKT cells after treatment with estrogen (10−8 M for 1 h). (C) AKT-mediated changes in ERα DNA-binding patterns to representative genes are shown. Open arrows indicate the direction of the gene in relation to chromosomal location, and black bars represent ERα binding sites in two cell types. (D) ERα binding pattern to chromosome 1 in two cell types with or without estrogen treatment.
FIG. 2.
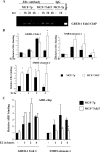
ChIP analysis of representative genes showing time-dependent changes in ERα and AIB1 recruitment in two cell types. (A) ChIP analysis of enhancer-3 region of GREB-1 for ERα binding. A total of 20 pg of ChIP DNA obtained using ERα antibody or control IgG was subjected to 30 cycles of PCR using GREB-1 enhancer-3-specific primers. (B) ChIP-Q-PCR analysis of ERα binding to select genes. GREB-1 enhancer-3 was analyzed again using ChIP DNA from independent experiments. Averages and standard deviations from three to five experiments are shown. ERα bound to SMRT-element-2 in MCF-7AKT cells in an estrogen-independent manner. Also, there was a difference in the duration of ERα binding to GREB-1 Enh-3 in the two cell types. *, P < 0.05 MCF-7p versus MCF-7AKT for the indicated time point. (C) Effect of AKT on recruitment of AIB1 to ERα binding regions of GREB-1 enhancer-3 and SMRT gene region 1. *, P = 0.02 (MCF-7p versus MCF-7AKT).
FIG. 3.
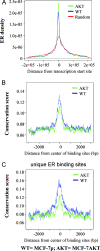
Sequence conservation and ER binding site distribution pattern in two cell types in relation to transcription start sites. (A) ERα binding site density in relation to the transcription start site. (B) Sequence conservation of all ERα binding sites in both cell types. Sequences flanking ERα binding regions are not conserved. (C) Sequence conservation of ERα binding sites that are unique to both cell types. Note the higher conservation of ERα binding sites in MCF-7p cells compared to MCF-7AKT cells (P < 10−16).
FIG. 4.
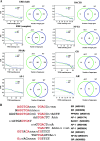
Enriched motifs within ERα ChIP-enriched regions in two cell types. (A) Enriched motifs in ERα binding region in two cell types. The y axes in the left panel demonstrate the ratio of the numbers of specific binding sites in the ChIP-enriched regions to the ones in randomly selected promoter sequences (1,000-bp upstream transcription starting site of the genes that are not ERα targets) or the FDR at different matching score cutoffs. The numbers in the Venn diagrams are calculated based on the matching score cutoffs that results in a 20% FDR. Motif enrichment was similar in both cell types, even within binding sites that are unique to a cell type. (B) Alignment of enriched motifs with the ERE. Nucleotide sequences indicated in red are shared with ERE.
FIG. 5.
Estrogen-regulated gene expression pattern in MCF-7p and MCF-7AKT cells. (A) Semiquantitative RT-PCR analyses of representative genes, which are associated with differential ERα binding after estrogen treatment in two cell types. Representative data from two or more experiments of a set of estrogen-inducible and estrogen-repressible genes are shown. (B) Quantitative RT-PCR analysis of representative genes in two cell types treated with estrogen for the indicated times. The data from three experiments, each performed in duplicate, are shown. PCRs contained water as a negative control, and no amplification was observed in the absence of cDNA in the reactions.
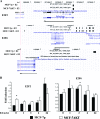
FIG. 6.
AKT overexpression leads to changes in estrogen-regulated expression of E2F2 and E2F6, which mediates secondary estrogen target gene expression. (A) ERα binding to E2F2 and E2F6 genes in two cell types. Two versions of E2F6 gene are shown: an expanded version depicting both E2F6 and the adjoining GREB-1 genes and a concise version with only E2F6 gene displaying the ERα binding site in MCF-7p cells. GREB-1 is an estrogen-regulated gene with multiple ERα binding sites. The direction of the gene (large arrow) and the ERα binding regions (black bars) are shown. (B) The basal and estrogen-inducible expression of E2F2 and E2F6 in MCF-7p and MCF-7AKT cells was measured by quantitative RT-PCR. The data from three experiments done in duplicate are shown (the asterisk indicates a comparison between MCF7p and MCF7AKT cells under similar treatment conditions [P < 0.05]).
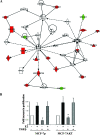
FIG. 7.
AKT reduces the growth-inhibitory function of TGF-β1. (A) Ingenuity pathway analysis of genes that are differentially expressed in estrogen-treated MCF-7p and MCF-7AKT cells. Genes indicated in green are repressed by estrogen, and those indicated in red are induced by estrogen in either or both cell types. (B) The effect of TGF-β1 on proliferation. Cells were treated with TGF-β (25 ng/ml) with or without estrogen (10−10 M) for 3 days, and the cell proliferation was measured by bromodeoxyuridine-enzyme-linked immunosorbent assay. Averages and standard deviations from four independent experiments are shown (TGF-β-treated MCF-7p cells versus TGF-β-treated MCF-7AKT cells [*, P = 0.01]).
Similar articles
- AKT3 regulates ErbB2, ErbB3 and estrogen receptor α expression and contributes to endocrine therapy resistance of ErbB2(+) breast tumor cells from Balb-neuT mice.
Grabinski N, Möllmann K, Milde-Langosch K, Müller V, Schumacher U, Brandt B, Pantel K, Jücker M. Grabinski N, et al. Cell Signal. 2014 May;26(5):1021-9. doi: 10.1016/j.cellsig.2014.01.018. Epub 2014 Jan 24. Cell Signal. 2014. PMID: 24463007 - A genome-wide study of the repressive effects of estrogen receptor beta on estrogen receptor alpha signaling in breast cancer cells.
Williams C, Edvardsson K, Lewandowski SA, Ström A, Gustafsson JA. Williams C, et al. Oncogene. 2008 Feb 7;27(7):1019-32. doi: 10.1038/sj.onc.1210712. Epub 2007 Aug 13. Oncogene. 2008. PMID: 17700529 - SIRT1 represses estrogen-signaling, ligand-independent ERα-mediated transcription, and cell proliferation in estrogen-responsive breast cells.
Moore RL, Faller DV. Moore RL, et al. J Endocrinol. 2013 Feb 15;216(3):273-85. doi: 10.1530/JOE-12-0102. Print 2013 Mar. J Endocrinol. 2013. PMID: 23169992 Free PMC article. - Estrogen receptor alpha phosphorylation and its functional impact in human breast cancer.
Anbalagan M, Rowan BG. Anbalagan M, et al. Mol Cell Endocrinol. 2015 Dec 15;418 Pt 3:264-72. doi: 10.1016/j.mce.2015.01.016. Epub 2015 Jan 15. Mol Cell Endocrinol. 2015. PMID: 25597633 Review. - The first decade of estrogen receptor cistromics in breast cancer.
Flach KD, Zwart W. Flach KD, et al. J Endocrinol. 2016 May;229(2):R43-56. doi: 10.1530/JOE-16-0003. Epub 2016 Feb 23. J Endocrinol. 2016. PMID: 26906743 Review.
Cited by
- Metabolic Reprogramming in Breast Cancer and Its Therapeutic Implications.
Gandhi N, Das GM. Gandhi N, et al. Cells. 2019 Jan 26;8(2):89. doi: 10.3390/cells8020089. Cells. 2019. PMID: 30691108 Free PMC article. Review. - Lrig1 is an estrogen-regulated growth suppressor and correlates with longer relapse-free survival in ERα-positive breast cancer.
Krig SR, Frietze S, Simion C, Miller JK, Fry WH, Rafidi H, Kotelawala L, Qi L, Griffith OL, Gray JW, Carraway KL 3rd, Sweeney C. Krig SR, et al. Mol Cancer Res. 2011 Oct;9(10):1406-17. doi: 10.1158/1541-7786.MCR-11-0227. Epub 2011 Aug 5. Mol Cancer Res. 2011. PMID: 21821674 Free PMC article. - Influence of AKT on progesterone action in endometrial diseases.
Lee II, Kim JJ. Lee II, et al. Biol Reprod. 2014 Sep;91(3):63. doi: 10.1095/biolreprod.114.119255. Epub 2014 Aug 6. Biol Reprod. 2014. PMID: 25100707 Free PMC article. Review. - Update on the Role of NFκB in Promoting Aggressive Phenotypes of Estrogen Receptor-Positive Breast Cancer.
Smart E, Semina SE, Frasor J. Smart E, et al. Endocrinology. 2020 Oct 1;161(10):bqaa152. doi: 10.1210/endocr/bqaa152. Endocrinology. 2020. PMID: 32887995 Free PMC article. Review. - AKT1 Transcriptomic Landscape in Breast Cancer Cells.
George B, Gui B, Raguraman R, Paul AM, Nakshatri H, Pillai MR, Kumar R. George B, et al. Cells. 2022 Jul 25;11(15):2290. doi: 10.3390/cells11152290. Cells. 2022. PMID: 35892586 Free PMC article.
References
- Ali, S., and R. C. Coombes. 2002. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer 2101-112. - PubMed
- Anzick, S. L., J. Kononen, R. L. Walker, D. O. Azorsa, M. M. Tanner, X. Y. Guan, G. Sauter, O. P. Kallioniemi, J. M. Trent, and P. S. Meltzer. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277965-968. - PubMed
- Auboeuf, D., A. Honig, S. M. Berget, and B. W. O'Malley. 2002. Coordinate regulation of transcription and splicing by steroid receptor coregulators. Science 298416-419. - PubMed
- Bellacosa, A., D. de Feo, A. K. Godwin, D. W. Bell, J. Q. Cheng, D. A. Altomare, M. Wan, L. Dubeau, G. Scambia, V. Masciullo, et al. 1995. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int. J. Cancer 64280-285. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous