Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents - PubMed (original) (raw)
. 2008 Oct 6;183(1):101-16.
doi: 10.1083/jcb.200801099.
Ann De Mazière, Christine Orr, Jie Lin, Brian B Lee, Janet Y Tien, Wei W Prior, Suzanne van Dijk, Hong Wu, Daniel C Gray, David P Davis, Howard M Stern, Lesley J Murray, Klaus P Hoeflich, Judith Klumperman, Lori S Friedman, Kui Lin
Affiliations
- PMID: 18838554
- PMCID: PMC2557046
- DOI: 10.1083/jcb.200801099
Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents
Michael Degtyarev et al. J Cell Biol. 2008.
Abstract
Although Akt is known as a survival kinase, inhibitors of the phosphatidylinositol 3-kinase (PI3K)-Akt pathway do not always induce substantial apoptosis. We show that silencing Akt1 alone, or any combination of Akt isoforms, can suppress the growth of tumors established from phosphatase and tensin homologue-null human cancer cells. Although these findings indicate that Akt is essential for tumor maintenance, most tumors eventually rebound. Akt knockdown or inactivation with small molecule inhibitors did not induce significant apoptosis but rather markedly increased autophagy. Further treatment with the lysosomotropic agent chloroquine caused accumulation of abnormal autophagolysosomes and reactive oxygen species, leading to accelerated cell death in vitro and complete tumor remission in vivo. Cell death was also promoted when Akt inhibition was combined with the vacuolar H(+)-adenosine triphosphatase inhibitor bafilomycin A1 or with cathepsin inhibition. These results suggest that blocking lysosomal degradation can be detrimental to cancer cell survival when autophagy is activated, providing rationale for a new therapeutic approach to enhancing the anticancer efficacy of PI3K-Akt pathway inhibition.
Figures
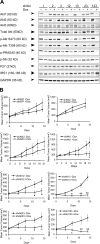
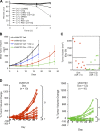
Figure 1.
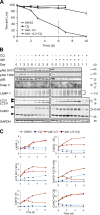
Inducible KD of Akt isoforms and their effect on xenograft tumor growth. (A) Immunoblot analysis of Akt isoforms and various downstream proteins in stable PC3 clones expressing the inducible shRNA constructs. Each clone was induced to express the respective shRNAs with 1 μg/ml Dox grown under 10% FBS for 7 d. Double arrowheads indicate slight differences in the mobility of the three Akt isoforms detected by total and phospho-Akt antibodies and the mobility shift of IRS1. (B) Effect of Akt KD on xenograft tumor growth. Representative experiments showing the growth of PC3 xenograft tumors containing the various shRNAs treated with vehicle control (−Dox, closed circles) or Dox (+Dox, open circles; see Table S2, available at
http://www.jcb.org/cgi/content/full/jcb.200801099/DC1
). Error bars represent SEM. *, P < 0.05; **, P < 0.005.
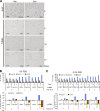
Figure 2.
Akt KD resulted in cell cycle delay without substantial apoptosis. (A) Histological analysis of PC3-shAkt123 tumors treated with Dox or vehicle control for 5, 15, or 21 d as indicated. Tumor tissues were analyzed by IHC using antibodies specific for Ki-67 or by the TUNEL assay. Pathologist's scoring of the signal intensity for each sample is indicated in parentheses. Bars, 100 μm. (B and C) Effect of triple-Akt KD on cell cycle progression under serum starvation (ss) compared with cells grown under 10% FBS. Cells containing shRNAs targeting EGFP or all three Akts were pretreated for 2 d with or without Dox in medium containing 10% FBS and changed to 0% (B) or 0.5% (C) FBS. Cell cycle profiles were analyzed at the indicated time points after serum withdrawal. Error bars represent SEM (n = 3). The percentage of change in each cell cycle phase with Dox versus without Dox treatment is also shown.
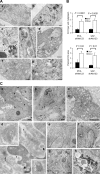
Figure 3.
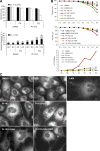
Autophagy was induced in PC3 and U87MG cells by Akt KD. (A) EM images of PC3 (a–d) and U87MG (e–g) cells grown in the absence (a and f) or presence (b–e and g) of Dox-induced Akt123 KD for 5 d. Arrows, degradative autolysosomes. Double arrows, initial AVs. Arrowhead, phagophore isolation membrane. M, mitochondrion in an AV. Asterisks, glycogen particle clusters. Bars: (a, b, f, and g) 0.5 μm; (c and d) 200 nm; (e) 1 μm. (B) Quantification of the number of AVs per unit cytoplasmic area of 4.5 μm2 (n ≥ 64) and the percentage of cytoplasmic area occupied by AV in randomly sampled cytoplasmic areas (n = 5 areas of >200 μm2) of PC3 and U87MG cells with and without Dox-induced shAkt123 expression. Error bars represent SEM. (C) Dox-induced Akt silencing caused degeneration in PC3 and U87MG tumors. (a) PC3 tumors expressing the control EGFP shRNA after 15 d of Dox treatment. The tumor cells contain large nuclei and nucleoli, some lipid droplets (asterisks), and are connected by cell junctions (arrowheads). (b–d) PC3 tumors expressing shAkt123 after 15 (b and c) or 10 d (d) of Dox treatment. (b) Cells and nuclei in these tumors often appear shrunken. Arrows, AVs. E, eosinophil. (c) Two AVs (arrows) found among dilated RER cisternae in a degenerating tumor cell. (d) Ultrathin cryosection with immunogold labeling of human LAMP1. Label occurs on lysosomes (arrow) and AVs (top inset). Some of the tumor cells also contain human LAMP1–positive dense bodies with a shape reminiscent of microautophagy (bottom inset; de Waal et al., 1986). The tumor cells have widened nuclear envelope and ER cisterns (asterisks), which contain small cytoplasmic islands (arrowheads). (e) U87MG tumor after 5 d of vehicle treatment. (f–h) U87MG-shAkt123 tumor after 5 d of Dox treatment. Arrows, AVs. (h) In some tumor samples, cells with glycogen clusters (asterisks) and glycogen-containing AVs occur. Bars: (a–c) 2 μm; (e and f) 1 μm; (g) 0.5 μm; (d and h) 200 nm.
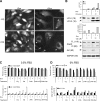
Figure 4.
Lysosomotropic agents accelerated cell death in combination with Akt KD. (A) CQ treatment caused accumulation of GFP-LC3 dots in Dox-treated PC3-shAkt123 cells. PC3-shAkt123 cells stably expressing GFP-LC3 were pretreated with or without 1 μg/ml Dox for 6 d and treated with or without 10 μM CQ. GFP fluorescence was imaged after 1 d of CQ treatment. Arrowheads point to representative GFP dots or clumps. Bar, 10 μm. (B) Effect of shAkt123 and 10 μM CQ on LC3 processing, PARP cleavage, and total Akt in PC3-shAkt123 cells treated with or without Dox or CQ. The ratios of LC3-II to LC3-I and cleaved (Cl) to full-length (FL) PARP were quantified from immunoblots of cell lysates made at days 1 and 2 of CQ treatment. Immunoblots of day 2 samples are shown. Molecular masses are indicated in kilodaltons parenthetically next to each protein. Data are representative of three independent experiments. (C) CQ promoted cell death in PC3 cells induced to express shAkt123, whereas 3-MA pretreatment delayed this effect. PC3-shAkt123 cells were preincubated with 1 μg/ml Dox and/or 1 mM 3-MA for 3 d before 10 μM CQ or 2.5 nM Ba was added. Cell viability was determined at days 2, 3, and 4 under 0.5% (C) or 0% (D) FBS after CQ or Ba was added. The percentage of the annexin V–positive PI-negative population was determined at days 2, 3, and 4 under 0.5% FBS. Caspase-3/7 activity was determined at days 2 and 3 under 0% FBS and expressed as relative fluorescence units (RFU, in thousands) normalized to the same number of cells. Error bars represent SD of three independent experiments.
Figure 5.
CQ accelerated cell death in combination with PI-103. (A) PC3 cells were treated with DMSO or 0.5 μM PI-103 in the presence or absence of 10 μM CQ under 0.5% FBS. Cell viability was determined by PI exclusion at days 2, 3, and 5. Annexin V staining was analyzed at days 2 and 3 and broken down into PI+ or PI− populations. (B) Time course of cell viability in PC3 cells treated with 0.5 (PI-103-0.5) or 20 μM (PI-103-20) PI-103 with or without 10 μM CQ or 3 mM 3-MA. PC3 cells pretreated with PI-103 for 24 h under 1% FBS were split into medium containing 0.5% FBS in the presence or absence of CQ. 3-MA was added immediately before PI-103, 24 h before CQ addition. Cell viability was determined by PI exclusion at the indicated time points after CQ addition. Error bars represent SEM (n = 3). LC3-II to LC3-I ratios were determined from quantitation of immunoblots (with 0.5 μM PI = 103. (C) CQ dramatically increased the size and number of MDC+ vacuoles in PC3 cells treated with PI-103, whereas 3-MA suppressed this effect. Cells were cultured in medium containing 0.5% FBS and treated with DMSO, 0.5 μM PI-103, 10 μM CQ, and 5 mM 3-MA, alone or in combinations as indicated. MDC staining at 48 h is shown. Bar, 10 μm.
Figure 6.
CQ accelerated cell death in combination with Akti-1/2. (A) PC3 cells were treated with DMSO or 4 μM Akti-1/2 in the presence or absence of 10 μM CQ under 0.5% FBS. Cell viability was determined by PI exclusion over the course of 10 d. Error bars represent SEM. Representative data from one of three independent experiments are shown. (B) Immunoblot analysis of cell lysates collected at the indicated time points from the experiment shown in A. Arrowheads indicate the positions for LC3-I and -II, CathD 43, and CathD 28. Quantifications of the indicated markers are shown in C. CathD 43, the 43–50-kD forms of cathepsin D precursors. CathD 28, the 28-kD cathepsin D heavy chain.
Figure 7.
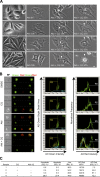
Accumulation of AVOs preceded plasma membrane rupture and correlated with the appearance of apoptotic and anucleated cells with Akti-1/2 and CQ treatment. (A) PC3 cells treated with DMSO, 10 μM Akti-1/2, 10 μM CQ, or both under 5% FBS were followed for 3 d using time-lapse microscopy. Representative images of the cells at the indicated time points are shown. White arrowheads indicate the fusion between two adjacent cells before plasma membrane rupture in cells treated with both agents. Full time-lapse videos are available in Videos 1–4, available at
http://www.jcb.org/cgi/content/full/jcb.200801099/DC1
. Bar, 10 μm. (B) PC3 cells treated with the indicated agents were stained with AO and analyzed by multispectral imaging flow cytometry. (left) Brightfield (BF), nuclei (green), vacuoles (red), and green/red composite images of three representative cells with each treatment are shown. Bars, 10 μm. (middle) plotting AO green intensity versus AO green bright detail area revealed three distinct populations: R2 anucleated cells, R3 apoptotic cells, and R4 live cells. (right) AO red intensity for R4 is plotted on the histogram with an arbitrary gate (R5) drawn to include events with the brightest AO red intensity. R2, R3, and R4 histograms are overlaid in the Akti + CQ plot only. (C) Statistics for each population shown in B. *, percentage of total single cells; **, mean fluorescence intensity of R4 live cells; ***, percentage of R4 live cells.
Figure 8.
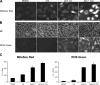
Akt inhibition induces mitochondrial superoxide and cellular ROS production, which is augmented by CQ. (A) PC3 cells cultured in 0.5% FBS were treated with DMSO, 3 μM Akti-1/2, 10 μM CQ, or both, stained with MitoSOX red dye, and examined by fluorescence microscopy. Images at 24 h are shown. Bar, 10 μm. (B) PC3 cells treated as in A were stained with the Image-iT LIVE green ROS Detection kit and examined by fluorescence microscopy at 24 h. Bright field (BF) images of cells are also shown. Bar, 10 μm. (C) Quantification of MitoSOX red and ROS green fluorescence intensities by flow cytometry at 24 h. Cells were treated as in A and B. Error bars represent SEM (n = 3).
Figure 9.
CQ selectively accelerated cell death in Akti-treated PTEN-null cells in vitro and enhanced the antitumor efficacy of Akt KD in vivo. (A) PTEN−/− (−/−) MEFs were more sensitive than isogenic PTEN+/+ (+/+) counterparts to the combined treatment with Akti-1/2 and CQ. MEFs were treated with 5 μM each of Akti-1/2 and CQ under 1% FBS, and cell viability was determined at days 0, 2, and 3 by PI exclusion. Error bars represent SEM (n = 3). (B) Mean tumor volumes of PC3 xenograft tumors treated daily with vehicle (Veh), Dox only, CQ only, or both Dox and CQ over a 28-d period. The vehicle and vehicle + CQ groups were followed for up to 18 d before terminated because of weight loss from the tumor burdens. Error bars represent SEM (n = 10 tumors in each cohort). (C) Scatterplot of the tumor volumes in the Dox only and Dox + CQ groups on day 28 (P = 0.05). Horizontal bars indicate mean tumor volumes. Numbers of tumors with complete remission (CR, dashed line) are indicated for each group. (D) Individual tumor growth plotted as a percentage of tumor volume change compared with day 0 for the Dox only and Dox + CQ cohorts shown in B. Dashed lines indicate −100% change from the starting tumor volumes, i.e., complete tumor regression. Numbers of tumors with smaller (<0% change) or larger (>0% change) than the starting tumor volumes on day 28 are indicated.
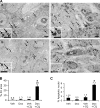
Figure 10.
Increased AV accumulation and apoptosis in PC3 tumor with combined Akt123 KD and CQ treatment. (A, a) EM images of PC3-shAkt123 tumors treated for 5 d with CQ only. Arrows, dense AVs and lysosomes; N, nucleolus. (b) Dox only. Arrows, AVs with a less dense appearance than in a. (c and d) Both Dox and CQ. (c) Numerous dense and enlarged AVs (arrows) accumulate in tumor cells. An apoptotic cell (Ap) is partially surrounded by a macrophage (M). T, tumor cell. (d) Apoptotic nuclei (Ap) among the AV-loaded (arrows) tumor cells. Insets, enlarged images of AVs (a–c) and abnormal mitochondria (*) in each tumor. Bars: (a–c) 2 μm; (d) 1 μm. (B) Quantification of the percentage of cytoplasmic area occupied by AVs in randomly sampled cytoplasmic areas (n = 6 areas of >80 μm2). (C) Percentage of apoptotic nuclei among randomly sampled tumor cell nuclei (n = 3–4 sets of 100 tumor cell nuclei). (B and C) Error bars represent SEM; *, P < 0.0005 compared with the other three groups.
Similar articles
- Blocked autophagy using lysosomotropic agents sensitizes resistant prostate tumor cells to the novel Akt inhibitor AZD5363.
Lamoureux F, Thomas C, Crafter C, Kumano M, Zhang F, Davies BR, Gleave ME, Zoubeidi A. Lamoureux F, et al. Clin Cancer Res. 2013 Feb 15;19(4):833-44. doi: 10.1158/1078-0432.CCR-12-3114. Epub 2012 Dec 20. Clin Cancer Res. 2013. PMID: 23258740 - Akt and autophagy cooperate to promote survival of drug-resistant glioma.
Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T, Haas-Kogan D, James CD, Oakes SA, Debnath J, Shokat KM, Weiss WA. Fan QW, et al. Sci Signal. 2010 Nov 9;3(147):ra81. doi: 10.1126/scisignal.2001017. Sci Signal. 2010. PMID: 21062993 Free PMC article. - Pro-apoptotic and pro-autophagic effects of the Aurora kinase A inhibitor alisertib (MLN8237) on human osteosarcoma U-2 OS and MG-63 cells through the activation of mitochondria-mediated pathway and inhibition of p38 MAPK/PI3K/Akt/mTOR signaling pathway.
Niu NK, Wang ZL, Pan ST, Ding HQ, Au GH, He ZX, Zhou ZW, Xiao G, Yang YX, Zhang X, Yang T, Chen XW, Qiu JX, Zhou SF. Niu NK, et al. Drug Des Devel Ther. 2015 Mar 12;9:1555-84. doi: 10.2147/DDDT.S74197. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25792811 Free PMC article. - Enhancing perifosine's anticancer efficacy by preventing autophagy.
Sun SY. Sun SY. Autophagy. 2010 Jan;6(1):184-5. doi: 10.4161/auto.6.1.10816. Epub 2010 Jan 1. Autophagy. 2010. PMID: 20023422 Review. - Autophagy, bafilomycin and cell death: the "a-B-cs" of plecomacrolide-induced neuroprotection.
Shacka JJ, Klocke BJ, Roth KA. Shacka JJ, et al. Autophagy. 2006 Jul-Sep;2(3):228-30. doi: 10.4161/auto.2703. Epub 2006 Jul 16. Autophagy. 2006. PMID: 16874105 Review.
Cited by
- Role for the phosphatidylinositol 3-kinase-Akt-TOR pathway during sindbis virus replication in arthropods.
Patel RK, Hardy RW. Patel RK, et al. J Virol. 2012 Apr;86(7):3595-604. doi: 10.1128/JVI.06625-11. Epub 2012 Jan 18. J Virol. 2012. PMID: 22258238 Free PMC article. - The regulatory role of NF-κB in autophagy-like cell death after focal cerebral ischemia in mice.
Li WL, Yu SP, Chen D, Yu SS, Jiang YJ, Genetta T, Wei L. Li WL, et al. Neuroscience. 2013 Aug 6;244:16-30. doi: 10.1016/j.neuroscience.2013.03.045. Epub 2013 Apr 1. Neuroscience. 2013. PMID: 23558089 Free PMC article. - Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation.
Ives A, Nomura J, Martinon F, Roger T, LeRoy D, Miner JN, Simon G, Busso N, So A. Ives A, et al. Nat Commun. 2015 Mar 24;6:6555. doi: 10.1038/ncomms7555. Nat Commun. 2015. PMID: 25800347 Free PMC article. - Caffeine induces apoptosis by enhancement of autophagy via PI3K/Akt/mTOR/p70S6K inhibition.
Saiki S, Sasazawa Y, Imamichi Y, Kawajiri S, Fujimaki T, Tanida I, Kobayashi H, Sato F, Sato S, Ishikawa K, Imoto M, Hattori N. Saiki S, et al. Autophagy. 2011 Feb;7(2):176-87. doi: 10.4161/auto.7.2.14074. Epub 2011 Feb 1. Autophagy. 2011. PMID: 21081844 Free PMC article. - Inhibition of autophagy and induction of glioblastoma cell death by NEO214, a perillyl alcohol-rolipram conjugate.
Ou M, Cho HY, Fu J, Thein TZ, Wang W, Swenson SD, Minea RO, Stathopoulos A, Schönthal AH, Hofman FM, Tang L, Chen TC. Ou M, et al. Autophagy. 2023 Dec;19(12):3169-3188. doi: 10.1080/15548627.2023.2242696. Epub 2023 Aug 6. Autophagy. 2023. PMID: 37545052 Free PMC article.
References
- Altomare, D.A., and J.R. Testa. 2005. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 24:7455–7464. - PubMed
- Arico, S., A. Petiot, C. Bauvy, P.F. Dubbelhuis, A.J. Meijer, P. Codogno, and E. Ogier-Denis. 2001. The tumor suppressor PTEN positively regulates macroautophagy by inhibiting the phosphatidylinositol 3-kinase/protein kinase B pathway. J. Biol. Chem. 276:35243–35246. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous