The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription - PubMed (original) (raw)
The variable immunological self: genetic variation and nongenetic noise in Aire-regulated transcription
Emily S Venanzi et al. Proc Natl Acad Sci U S A. 2008.
Abstract
The Aire transcription factor plays an important role in immunological self-tolerance by mediating the ectopic expression of peripheral self-antigens by thymic medullary epithelial cells (MECs), and the deletion of thymocytes that recognize them. In Aire-deficient humans or mice, central tolerance is incomplete and multiorgan autoimmune disease results. We examined the variability of Aire's effects on ectopic transcription among individual mice of three different inbred strains. Aire's function was, overall, quite similar in the three backgrounds, although generally stronger in C57BL/6 than in BALB/c or NOD mice, and a minority of Aire-regulated genes did show clear differences. Gene expression profiling of wild-type MECs from single mice, or from the two thymic lobes of the same mouse, revealed significantly greater variability in Aire-controlled ectopic gene expression than in Aire-independent transcripts. This "noisy" ectopic expression did not result from parental or early developmental imprinting, but from programming occurring after the formation of the thymic anlage, resulting from epigenetic effects or from the stochastic nature of Aire activity. Together, genetic and nongenetic variability in ectopic expression of peripheral antigens in the thymus make for differences in the portion of self determinants presented for tolerance induction. This variable self may be beneficial in preventing uniform holes in the T-cell repertoire in individuals of a species, but at the cost of variable susceptibility to autoimmunity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Similarities of Aire effects on gene expression in MECs from mice with different genetic backgrounds. Gene expression profiles were determined for MECs of mice from three inbred lines. The scatter plots display gene expression values (averaged from three replicates per strain) for all probes on the M430 array, comparing expression in KOs and WT littermate controls. Red and green dots indicate probes with a WT/KO FC value >2, or <0.5, in all 3 strains.
Fig. 2.
Fine differences in Aire's regulation of gene expression in different genetic backgrounds. (A) Comparisons of WT/KO ratios in MECs from the three strains. The red line on each plot shows the results of a locally smoothed regression (lowess). The average slope derived from the lowess is shown on each graph. (B) Histograms of the difference between FC values determined for Aire-induced or Aire-repressed genes, comparing strains pairwise. These differences were calculated for each gene by subtracting the log2(FC) values.
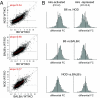
Fig. 3.
Aire protein levels do not vary among strains. Flow cytometric analysis of Aire expression in MECs of different genetic backgrounds (Aire vs MHC-IIhi profiles of gated CD45−G8.8+Ly51intMHC-IIhi MECs; representative of three independent mice for each inbred strain). Numbers are the mean percentage of Aire+ cells among class IIhi MECs, and the mean anti-Aire fluorescence intensity, ± SD, of Aire staining.
Fig. 4.
Expression of Aire-regulated genes varies among individuals. (A) CV vs. FC plots of all genes on the M430 array, from the expression values in the three B6 WT (Left) or B6 KO (Right) individual mice. Also indicated on each plot are the mean CVs of Aire-activated transcripts (WT/KO FC >2), Aire-repressed (WT/KO FC <0.5), or Aire-neutral (WT/KO FC between 0.77 and 1.3 and chosen to have the same distribution of expression values as the combined Aire-activated and Aire-repressed lists). Analysis of the BALB/c and NOD strains yielded similar results (Table 2). (_B_) Histograms of CVs for Aire-activated (red), Aire-repressed (green), and Aire-neutral (black) probes in WT (_Top_) and KO _(Lower)_ MECs. (_C_) Scatter plot comparing CVs in M430 datasets from liver or MECs (B6 strain). Red and green dots are probes with a WT/KO FC value >2, or <0.5, in all three strains. (_D_) Expression values for several Aire-activated and Aire-neutral genes. Each point represents the expression level in an individual mouse, with shapes indicating the different strains. The Aire-activated genes shown are representative of those genes having a WT/KO FC >2 in MECs, and an interindividual CV of at least 0.9. Aire-neutral genes had a WT/KO FC between 0.77 and 1.3 and an interindividual CV at the median, 0.16.
Fig. 5.
Patterns of Aire-induced expression vary independently in each thymic lobe. MECs were purified from left and right thymic lobes of four wild-type B6 mice, and each (A) Histogram of genewise CVs across all datasets, for Aire-induced genes (FC >2.5) or all other genes on the array. (B) Relative Euclidian distances were calculated between each individual lobe, integrating expression values for all Aire-induced genes.
Similar articles
- Global relevance of Aire binding to hypomethylated lysine-4 of histone-3.
Koh AS, Kingston RE, Benoist C, Mathis D. Koh AS, et al. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13016-21. doi: 10.1073/pnas.1004436107. Epub 2010 Jul 6. Proc Natl Acad Sci U S A. 2010. PMID: 20615959 Free PMC article. - Ectopic Aire Expression in the Thymic Cortex Reveals Inherent Properties of Aire as a Tolerogenic Factor within the Medulla.
Nishijima H, Kitano S, Miyachi H, Morimoto J, Kawano H, Hirota F, Morita R, Mouri Y, Masuda K, Imoto I, Ikuta K, Matsumoto M. Nishijima H, et al. J Immunol. 2015 Nov 15;195(10):4641-9. doi: 10.4049/jimmunol.1501026. Epub 2015 Oct 9. J Immunol. 2015. PMID: 26453754 - Projection of an immunological self shadow within the thymus by the aire protein.
Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Anderson MS, et al. Science. 2002 Nov 15;298(5597):1395-401. doi: 10.1126/science.1075958. Epub 2002 Oct 10. Science. 2002. PMID: 12376594 - AIRE: From promiscuous molecular partnerships to promiscuous gene expression.
Abramson J, Goldfarb Y. Abramson J, et al. Eur J Immunol. 2016 Jan;46(1):22-33. doi: 10.1002/eji.201545792. Epub 2015 Nov 2. Eur J Immunol. 2016. PMID: 26450177 Review. - Central tolerance to self revealed by the autoimmune regulator.
Chan AY, Anderson MS. Chan AY, et al. Ann N Y Acad Sci. 2015 Nov;1356(1):80-9. doi: 10.1111/nyas.12960. Ann N Y Acad Sci. 2015. PMID: 26579596 Free PMC article. Review.
Cited by
- Global relevance of Aire binding to hypomethylated lysine-4 of histone-3.
Koh AS, Kingston RE, Benoist C, Mathis D. Koh AS, et al. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13016-21. doi: 10.1073/pnas.1004436107. Epub 2010 Jul 6. Proc Natl Acad Sci U S A. 2010. PMID: 20615959 Free PMC article. - AIRE in the thymus and beyond.
Gardner JM, Fletcher AL, Anderson MS, Turley SJ. Gardner JM, et al. Curr Opin Immunol. 2009 Dec;21(6):582-9. doi: 10.1016/j.coi.2009.08.007. Epub 2009 Oct 14. Curr Opin Immunol. 2009. PMID: 19833494 Free PMC article. Review. - The role of AIRE in human autoimmune disease.
Akirav EM, Ruddle NH, Herold KC. Akirav EM, et al. Nat Rev Endocrinol. 2011 Jan;7(1):25-33. doi: 10.1038/nrendo.2010.200. Epub 2010 Nov 23. Nat Rev Endocrinol. 2011. PMID: 21102544 Review. - Twenty Years of AIRE.
Perniola R. Perniola R. Front Immunol. 2018 Feb 12;9:98. doi: 10.3389/fimmu.2018.00098. eCollection 2018. Front Immunol. 2018. PMID: 29483906 Free PMC article. Review. - Aire and T cell development.
Anderson MS, Su MA. Anderson MS, et al. Curr Opin Immunol. 2011 Apr;23(2):198-206. doi: 10.1016/j.coi.2010.11.007. Epub 2010 Dec 14. Curr Opin Immunol. 2011. PMID: 21163636 Free PMC article. Review.
References
- Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–1039. - PubMed
- Heino M, et al. Autoimmune regulator is expressed in the cells regulating immune tolerance in thymus medulla. Biochem Biophys Res Commun. 1999;257:821–825. - PubMed
- Heino M, et al. RNA and protein expression of the murine autoimmune regulator gene (Aire) in normal, RelB-deficient and in NOD mouse. Eur J Immunol. 2000;30:1884–1893. - PubMed
- Anderson MS, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK060027/DK/NIDDK NIH HHS/United States
- T32 DK007260/DK/NIDDK NIH HHS/United States
- T32 DK07260/DK/NIDDK NIH HHS/United States
- R01 DK60027/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases