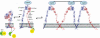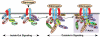The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend - PubMed (original) (raw)
Review
The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend
Barry S Coller et al. Blood. 2008.
Abstract
Starting 90 years ago with a clinical description by Glanzmann of a bleeding disorder associated with a defect in platelet function, technologic advances helped investigators identify the defect as a mutation(s) in the integrin family receptor, alphaIIbbeta3, which has the capacity to bind fibrinogen (and other ligands) and support platelet-platelet interactions (aggregation). The receptor's activation state was found to be under exquisite control, with activators, inhibitors, and elaborate inside-out signaling mechanisms controlling its conformation. Structural biology has produced high-resolution images defining the ligand binding site at the atomic level. Research on alphaIIbbeta3 has been bidirectional, with basic insights resulting in improved Glanzmann thrombasthenia carrier detection and prenatal diagnosis, assays to identify single nucleotide polymorphisms responsible for alloimmune neonatal thrombocytopenia, and the development of alphaIIbbeta3 antagonists, the first rationally designed antiplatelet agents, to prevent and treat thrombotic cardiovascular disease. The future looks equally bright, with the potential for improved drugs and the application of gene therapy and stem cell biology to address the genetic abnormalities. The alphaIIbbeta3 saga serves as a paradigm of rigorous science growing out of careful clinical observations of a rare disorder yielding both important new scientific information and improved diagnosis, therapy, and prevention of other disorders.
Figures
Figure 1
Timeline of application of new technologies to the study of platelets and/or αIIbβ3.
Figure 2
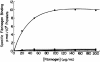
Platelet fibrinogen binding studies demonstrate that platelets from patients with Glanzmann thrombasthenia cannot bind fibrinogen in response to ADP stimulation. The upper curve is of platelets from a healthy subject and the 3 lower ones are from 3 different patients. Reprinted with permission from Bennett JS, Vilaire G. J Clin Invest. 1979;64:1393-1401.
Figure 3
Application of flow cytometry and the activation-dependent monoclonal antibody PAC-1 to the study of αIIbβ3 conformational changes and ligand binding. (A) Platelets were identified and differentiated from red and white blood cells by their characteristic forward and side-angle light scatter profiles. (B) Platelets were stimulated with ADP and epinephrine or incubated with PGI2 to block activation. The fluorescence histogram depicts biotin-PAC-1 binding to the platelets detected by phycoerythrin-streptavidin. Reprinted from Shattil et al. Blood. 1987;70:307.
Figure 4
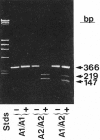
Application of reverse transcription and the polymerase chain reaction to identify the PlA1 polymorphism as due to a nucleotide mutation leading to a Leu33Pro substitution in the integrin β3 subunit. Bases 56-408 of integrin β3 were enzymatically amplified from individuals who were homozygous PlA2 or heterozygous PlA1/PlA2 and analyzed on agarose tells. The enzyme is sensitive to the T→C change in the sequence at base 196 associated with the PlA2 polymorphism. Reprinted with permission from Newman et al. J Clin Invest. 1989;83:1778-1781.
Figure 5
Model of αIIbβ3 based on αVβ3 crystal structure and depiction of switchblade model of αIIbβ3 conformational changes associated with activation and ligand binding. Inside-out signaling ultimately results in the binding of the talin head (H) domain binding to the cytoplasmic domain of the β3 subunit, resulting in subunit separation. This is transmitted through the transmembrane domains to the ectodomain where it results in extension of the α and β subunits and perhaps additional changes in the ligand binding region of β3. Ligand then binds, resulting in a swing-out motion of the β3 hybrid and PSI domains that may initiate outside-in signaling. Additional post-ligand binding events may occur, including homo-oligomerization of integrin transmembrane domains, leading to receptor clustering. The “deadbolt” hypothesis posits that modest changes in the β3 βA (I-like) domain brought about by movement of a nearby β3 β-terminal domain loop results in ligand binding, which is then followed by receptor extension and the swing-out motion. Adapted from Qin et al. The molecular models of αIIbβ3 were constructed using MODELLER 8v2 and the PDBs ITY6, IU8C, and IYUK as previously described. I-EGF, integrin epidermal growth factor domain; β-TD, β-terminal domain.
Figure 6
Protein interactions with the cytoplasmic domains of αIIb or β3 regulate integrin signaling. Shown are some, but not all, of the proteins reported to associate with the αIIbβ3 cytoplasmic domains, many in a dynamic fashion. Some associate with αIIbβ3 in resting platelets, while others are recruited to, or dissociate from, the integrin during inside-out or outside-in signaling, leading to F-actin assembly. In addition, several proteins with enzymatic function become activated (asterisk) after fibrinogen binding to αIIbβ3. It is difficult to imagine that all of these proteins can interact with a single αIIbβ3 heterodimer in platelets; however, they might interact with and further regulate oligomers of αIIbβ3 that form in response to fibrinogen binding. Not shown are the many additional adapter molecules, enzymes and substrates that may become recruited through more indirect interactions during the various phases of integrin signaling. Abbreviations: PP1c, protein phosphatase 1c; RACK1, receptor for activated C kinase 1; Csk, c-Src tyrosine kinase; PKCβ, protein kinase Cβ; ILK, integrin-linked kinase; ITAM, a yet-to-be-identified protein with one or more immunoreceptor tyrosine activation motifs; CIB, calcium and integrin-binding 1; Syk, spleen tyrosine kinase.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - The CalDAG-GEFI/Rap1/αIIbβ3 axis minimally contributes to accelerated platelet clearance in mice with constitutive store-operated calcium entry.
Lee RH, Rocco DJ, Nieswandt B, Bergmeier W. Lee RH, et al. Platelets. 2023 Dec;34(1):2157383. doi: 10.1080/09537104.2022.2157383. Platelets. 2023. PMID: 36683325 Free PMC article. - Unlocking data: Decision-maker perspectives on cross-sectoral data sharing and linkage as part of a whole-systems approach to public health policy and practice.
Tweed E, Cimova K, Craig P, Allik M, Brown D, Campbell M, Henderson D, Mayor C, Meier P, Watson N. Tweed E, et al. Public Health Res (Southampt). 2024 Nov 20:1-30. doi: 10.3310/KYTW2173. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39582242 - Trends in Surgical and Nonsurgical Aesthetic Procedures: A 14-Year Analysis of the International Society of Aesthetic Plastic Surgery-ISAPS.
Triana L, Palacios Huatuco RM, Campilgio G, Liscano E. Triana L, et al. Aesthetic Plast Surg. 2024 Oct;48(20):4217-4227. doi: 10.1007/s00266-024-04260-2. Epub 2024 Aug 5. Aesthetic Plast Surg. 2024. PMID: 39103642 Review.
Cited by
- One immune cell to bind them all: platelet contribution to neurodegenerative disease.
Rodriguez Moore G, Melo-Escobar I, Stegner D, Bracko O. Rodriguez Moore G, et al. Mol Neurodegener. 2024 Sep 27;19(1):65. doi: 10.1186/s13024-024-00754-4. Mol Neurodegener. 2024. PMID: 39334369 Free PMC article. Review. - No crops without seeds: the risks in declining support for fundamental research.
Hogan BM, Kahn ML, Harvey NL. Hogan BM, et al. Nat Cardiovasc Res. 2023 Mar;2(3):193-195. doi: 10.1038/s44161-023-00225-x. Nat Cardiovasc Res. 2023. PMID: 39195998 No abstract available. - Synthetic integrin antibodies discovered by yeast display reveal αV subunit pairing preferences with β subunits.
Hao Y, Yan J, Fraser C, Jiang A, Anuganti M, Zhang R, Lloyd K, Jardine J, Coppola J, Meijers R, Li J, Springer TA. Hao Y, et al. MAbs. 2024 Jan-Dec;16(1):2365891. doi: 10.1080/19420862.2024.2365891. Epub 2024 Jun 18. MAbs. 2024. PMID: 38889315 Free PMC article. - Synthetic integrin antibodies discovered by yeast display reveal αV subunit pairing preferences with β subunits.
Hao Y, Yan J, Fraser C, Jiang A, Anuganti M, Zhang R, Lloyd K, Jardine J, Coppola J, Meijers R, Li J, Springer TA. Hao Y, et al. bioRxiv [Preprint]. 2024 Jan 27:2024.01.26.577394. doi: 10.1101/2024.01.26.577394. bioRxiv. 2024. PMID: 38328192 Free PMC article. Updated. Preprint.
References
- Gabriele M. Blood and magic in classical antiquity. In: Bradburne JM, editor. Blood: Art, Power, Politics and Pathology. Munich: Prestel; 2002. pp. 33–39.
- Medical Meanings: A Glossary of Word Origins. Philadelphia: American College of Physicians; 1997.
- George JN, Nurden AT, Phillips DR, editors. Platelet membrane glycoproteins. New York: Plenum; 1985. - PubMed
- Michelson AD, editor. Platelets. Burlington, MA: Academic Press; 2007.
- Lichtman MA, Beutler E, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Hematology. New York: McGraw-Hill; 2006.
Publication types
MeSH terms
Substances
Grants and funding
- HL56595/HL/NHLBI NIH HHS/United States
- R03 MH083257/MH/NIMH NIH HHS/United States
- UL1 RR024143/RR/NCRR NIH HHS/United States
- MH083257/MH/NIMH NIH HHS/United States
- HL57900/HL/NHLBI NIH HHS/United States
- HL19278/HL/NHLBI NIH HHS/United States
- R01 HL019278/HL/NHLBI NIH HHS/United States
- P01 HL078784/HL/NHLBI NIH HHS/United States
- HL78784/HL/NHLBI NIH HHS/United States
- P01 HL057900/HL/NHLBI NIH HHS/United States
- R01 HL056595/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources