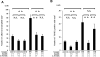Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage - PubMed (original) (raw)
doi: 10.2337/db08-0762. Epub 2008 Oct 7.
Yasuaki Hata, Shintaro Nakao, Takeshi Kita, Muneki Miura, Shuhei Kawahara, Souska Zandi, Lama Almulki, Faryan Tayyari, Hiroaki Shimokawa, Ali Hafezi-Moghadam, Tatsuro Ishibashi
Affiliations
- PMID: 18840783
- PMCID: PMC2606876
- DOI: 10.2337/db08-0762
Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage
Ryoichi Arita et al. Diabetes. 2009 Jan.
Abstract
Objective: Leukocyte adhesion in retinal microvasuculature substantially contributes to diabetic retinopathy. Involvement of the Rho/Rho kinase (ROCK) pathway in diabetic microvasculopathy and therapeutic potential of fasudil, a selective ROCK inhibitor, are investigated.
Research design and methods: Localization of RhoA/ROCK and Rho activity were examined in retinal tissues of rats. Impact of intravitreal fasudil administration on retinal endothelial nitric oxide synthase (eNOS) and myosin phosphatase target protein (MYPT)-1 phosphorylation, intercellular adhesion molecule-1 (ICAM-1) expression, leukocyte adhesion, and endothelial damage in rat eyes were investigated. Adhesion of neutrophils from diabetic retinopathy patients or nondiabetic control subjects to cultured microvascular endothelial cells was quantified. The potential of fasudil for endothelial protection was investigated by measuring the number of adherent neutrophils and terminal transferase-mediated dUTP nick-end labeling-positive endothelial cells.
Results: RhoA and ROCK colocalized predominantly in retinal microvessels. Significant Rho activation was observed in retinas of diabetic rats. Intravitreal fasudil significantly increased eNOS phosphorylation, whereas it reduced MYPT-1 phosphorylation, ICAM-1 expression, leukocyte adhesion, and the number of damaged endothelium in retinas of diabetic rats. Neutrophils from diabetic retinopathy patients showed significantly higher adhesion to cultured endothelium and caused endothelial apoptosis, which was significantly reduced by fasudil. Blockade of the Fas-FasL interaction prevented endothelial apoptosis. The protective effect of fasudil on endothelial apoptosis was significantly reversed by Nomega-nitro-l-arginine methyl ester, a NOS inhibitor, whereas neutrophil adhesion remained unaffected.
Conclusions: The Rho/ROCK pathway plays a critical role in diabetic retinal microvasculopathy. Fasudil protects the vascular endothelium by inhibiting neutrophil adhesion and reducing neutrophil-induced endothelial injury. ROCK inhibition may become a new strategy in the management of diabetic retinopathy, especially in its early stages.
Figures
FIG. 1.
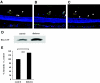
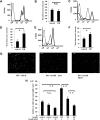
Localization and activity of Rho/ROCK in retinal vessels. Paraffin-embedded sections of nondiabetic rat retinas were immunohistochemically analyzed with RhoA (A), ROCK1 (B), and ROCK2 (C) antibodies (magnification ×400). Endothelial cells were stained with rhodamin-conjugated anti-CD34 (red). RhoA, ROCK1, and ROCK2 were detected by green fluorescence. Yellow (white arrowhead) indicates double-stained vasculature. D: Representative blot showing the level of ρ-GTP in nondiabetic control or STZ-induced diabetic rat retinas detected by Rho pull-down assay. E: Average signal intensities quantified and expressed as percentage of the ratio of control (**P < 0.01, n = 5 each). (Please see
http://dx.doi.org/10.2337/db08-0762
for a high-quality digital representation of this figure.)
FIG. 2.
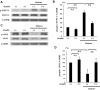
Impact of intravitreal fasudil on retinal ROCK activity. Phosphorylated MYPT-1 (Thr853) and eNOS (Ser1177) were detected in rat retinal preparations by Western blot analysis. Lane loading differences were normalized by reblotting the membranes with an antibody against GAPDH. A: Representative results of phospho-MYPT-1 and GAPDH in rat retinas. B: ROCK activity was expressed as the ratio of phospho-MYPT-1 to GAPDH (**P < 0.01, NS; n = 6 each). C: Representative results of phospho-eNOS and GAPDH. D: Average signal intensities quantified and expressed as percentage of the ratio of control (**P < 0.05, NS; n = 6 each).
FIG. 3.
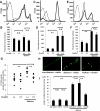
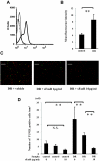
Reduction of diabetes-induced retinal leukocyte adhesion by fasudil. Intravitreal injections of fasudil at 0 μmol/l (vehicle) or 30 μmol/l (final vitreal concentration) were performed every 3 days for 2 weeks. Cell surface expressions of CD11a (A), CD11b (C), and CD18 (E) on nondiabetic control (thin line) and diabetic rat neutrophils (thick line) were analyzed by flow cytometry. Mean fluorescence intensity of CD11a (B), CD11b (D), and CD18 (F) (**P < 0.01, NS; n = 8 each; dotted line, mouse isotype control). G: Retinal ICAM-1 concentrations measured by enzyme-linked immunosorbent assay in retinas of normal and diabetic animals with and without fasudil treatment (**P < 0.01, n = 7 each). H: Representative ConA-stained flat mounts of normal and diabetic animals with and without fasudil treatment. White arrowhead, firmly adhering leukocytes. I: Quantitative analysis of the number of firmly adhering leukocytes in normal and diabetic rats with and without fasudil treatment (**P < 0.01, NS; n = 7 each). (Please see
http://dx.doi.org/10.2337/db08-0762
for a high-quality digital representation of this figure.)
FIG. 4.
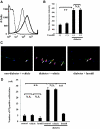
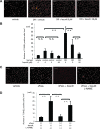
Prevention of neutrophil-induced retinal endothelial damage by fasudil. A: Cell surface expressions of FasL on nondiabetic control (thin line) and diabetic (thick line) rat neutrophils were analyzed by flow cytometry. B: Average mean fluorescence intensity of FasL on peripheral blood neutrophils from normal and diabetic rats with and without intravitreal fasudil injection (**P < 0.01, NS; n = 8 each; dotted line, mouse isotype control). C and D: In vivo visualization of dead or injured endothelial cells (red, propidium iodide [PI]) and endothelial nuclei (blue, DAPI) in rat retinas. Propidium iodide–positive cells (white arrowhead) widely coincided with adherent leukocytes (white arrow). The number of propidium iodide–positive cells per retina was significantly higher in the diabetic animals compared with the nondiabetic controls. Fasudil caused a significant reduction in the number of propidium iodide–positive cells in the retinas of the diabetic animals compared with the vehicle-treated controls (**P < 0.01, NS; n = 5 each). (Please see
http://dx.doi.org/10.2337/db08-0762
for a high-quality digital representation of this figure.)
FIG. 5.
Reduced adhesion of neutrophils from diabetic retinopathy patients to HMVECs by fasudil. Cell surface expressions of CD11a (A), CD11b (C), and CD18 (E) on neutrophils from nondiabetic control subjects (thin line) and those with diabetic retinopathy (DR) (thick line) were analyzed by flow cytometry. Mean fluorescence intensity of CD11a (B), CD11b (D), CD18 (F) (*P < 0.05, NS; n = 20 each; dotted line, mouse isotype control). G and H: Confluent HMVECs in collagen-coated 24-well plates were pretreated with 0, 5, or 20 μmol/l fasudil for 1 h and subsequently stimulated with 10 ng/ml rhTNF-α for 12 h. Labeled neutrophils (1 mmol/l Calcein-AM) were coincubated (2 × 105 cells/ml, 500 μl/well) with pretreated HMVECs for 1 h at 37°C. Scale bar = 100 μm. The number of adherent neutrophils in four different areas per well was counted and averaged (**P < 0.01, NS; n = 15 each).
FIG. 6.
Fas/FasL-mediated endothelial apoptosis by adherent neutrophils. A: Cell surface expressions of FasL on neutrophils from nondiabetic control subjects (thin line) and diabetic retinopathy (DR) patients (thick line) were analyzed by flow cytometry. B: Mean fluorescence intensity of FasL (**P < 0.05, n = 17 each; dotted line, mouse isotype control). C and D: HMVECs were labeled with 1 mmol/l Hoechst 33342 (red fluorescence). HMVECs were stimulated for 12 h with 10 ng/ml rhTNF-α. Neutrophils were incubated with 0, 1, and 10 μg/ml sFas receptor (sFasR) before coculture for 1 h. Subsequently, unlabeled neutrophils (5 × 105cells/ml) from nondiabetic control subjects or diabetic retinopathy patients were cocultured with HMVECs for 12 h. Apoptotic HMVECs demonstrated double labeling and appeared yellow. Scale bar = 100 μm. The number of TUNEL-positive cells in four different areas per well was counted and averaged (**P < 0.01, NS; n = 5 each). (Please see
http://dx.doi.org/10.2337/db08-0762
for a high-quality digital representation of this figure.)
FIG. 7.
Prevention of neutrophil-induced endothelial apoptosis by fasudil. A and B: After pretreatment with 0, 5, or 20 μmol/l fasudil for 1 h, HMVECs were stimulated for 12 h with 10 ng/ml rhTNF-α. Subsequently, unlabeled neutrophils (5 × 105cells/ml) were cocultured with HMVECs for 12 h. Scale bar = 100 μm. The number of apoptotic cells (yellow fluorescence) in four different areas per well was counted (**P < 0.01, NS; n = 15 each). C and D: Involvement of fasudil in sFasL-induced apoptosis was investigated. HMVECs were preincubated with or without 20 μmol/l fasudil before sFasL treatment for 1 h. Furthermore, HMVECs were incubated with or without 1 mmol/l
l
-NAME before fasudil treatment for 1 h. Scale bar = 100 μm. The number of TUNEL-positive cells in four different areas per well was counted and averaged (**P < 0.01, NS; n = 4 each). (Please see
http://dx.doi.org/10.2337/db08-0762
for a high-quality digital representation of this figure.)
FIG. 8.
Prevention of neutrophil-induced endothelial apoptosis by fasudil. After pretreatment with 0 or 20 μmol/l fasudil for 1 h, HMVECs were stimulated for 12 h with 10 ng/ml rhTNF-α. Subsequently, unlabeled neutrophils (5 × 105cells/ml) were cocultured with HMVECs for 12 h. The number of apoptotic cells (yellow fluorescence) in four different areas per well was counted and averaged. A and B: Impact of NO on neutrophil adhesion and endothelial apoptosis.
l
-NAME (0 or 1 mmol/l) was preincubated with HMVECs before fasudil treatment for 1 h. The total number of TUNEL-positive cells in four different areas per well was counted and averaged (**P < 0.01, NS; n = 10 each).
Similar articles
- [Mechanism of diabetes-induced microvascular damage and therapeutic potential of ROCK inhibition].
Arita R. Arita R. Nippon Ganka Gakkai Zasshi. 2011 Nov;115(11):985-97. Nippon Ganka Gakkai Zasshi. 2011. PMID: 22171504 Review. Japanese. - A key role for ROCK in TNF-α-mediated diabetic microvascular damage.
Arita R, Nakao S, Kita T, Kawahara S, Asato R, Yoshida S, Enaida H, Hafezi-Moghadam A, Ishibashi T. Arita R, et al. Invest Ophthalmol Vis Sci. 2013 Mar 28;54(3):2373-83. doi: 10.1167/iovs.12-10757. Invest Ophthalmol Vis Sci. 2013. PMID: 23462755 - Rho kinase inhibition by fasudil suppresses lipopolysaccharide-induced apoptosis of rat pulmonary microvascular endothelial cells via JNK and p38 MAPK pathway.
Liu H, Chen X, Han Y, Li C, Chen P, Su S, Zhang Y, Pan Z. Liu H, et al. Biomed Pharmacother. 2014 Apr;68(3):267-75. doi: 10.1016/j.biopha.2013.12.003. Epub 2013 Dec 24. Biomed Pharmacother. 2014. PMID: 24406296 - ROCK induced inflammation of the microcirculation during endotoxemia mediated by nitric oxide synthase.
McGown CC, Brown NJ, Hellewell PG, Brookes ZL. McGown CC, et al. Microvasc Res. 2011 May;81(3):281-8. doi: 10.1016/j.mvr.2011.02.003. Epub 2011 Feb 23. Microvasc Res. 2011. PMID: 21354186 - Could pharmacological curtailment of the RhoA/Rho-kinase pathway reverse the endothelial barrier dysfunction associated with Ebola virus infection?
Eisa-Beygi S, Wen XY. Eisa-Beygi S, et al. Antiviral Res. 2015 Feb;114:53-6. doi: 10.1016/j.antiviral.2014.12.005. Epub 2014 Dec 13. Antiviral Res. 2015. PMID: 25512227 Review.
Cited by
- The role of Rho/Rho-kinase pathway in the expression of ICAM-1 by linoleic acid in human aortic endothelial cells.
Jung CH, Lee WJ, Hwang JY, Seol SM, Kim YM, Lee YL, Ahn JH, Park JY. Jung CH, et al. Inflammation. 2012 Jun;35(3):1041-8. doi: 10.1007/s10753-011-9409-2. Inflammation. 2012. PMID: 22124782 - Update on Management of Non-proliferative Diabetic Retinopathy without Diabetic Macular Edema; Is There a Paradigm Shift?
Arabi A, Tadayoni R, Ahmadieh H, Shahraki T, Nikkhah H. Arabi A, et al. J Ophthalmic Vis Res. 2022 Jan 21;17(1):108-117. doi: 10.18502/jovr.v17i1.10175. eCollection 2022 Jan-Mar. J Ophthalmic Vis Res. 2022. PMID: 35194501 Free PMC article. Review. - Molecular and clinical aspects of endothelial dysfunction in diabetes.
Nacci C, Tarquinio M, Montagnani M. Nacci C, et al. Intern Emerg Med. 2009 Apr;4(2):107-16. doi: 10.1007/s11739-009-0234-7. Epub 2009 Mar 10. Intern Emerg Med. 2009. PMID: 19280353 - Addition of ROCK inhibitors to prostaglandin derivative (PG) synergistically affects adipogenesis of the 3D spheroids of human orbital fibroblasts (HOFs).
Hikage F, Ichioka H, Watanabe M, Umetsu A, Ohguro H, Ida Y. Hikage F, et al. Hum Cell. 2022 Jan;35(1):125-132. doi: 10.1007/s13577-021-00623-y. Epub 2021 Sep 30. Hum Cell. 2022. PMID: 34591280 - Ocular effects of Rho kinase (ROCK) inhibition: a systematic review.
Lin JB, Harris JM, Baldwin G, Goss D, Margeta MA. Lin JB, et al. Eye (Lond). 2024 Dec;38(18):3418-3428. doi: 10.1038/s41433-024-03342-4. Epub 2024 Sep 16. Eye (Lond). 2024. PMID: 39285241 Review.
References
- Pastor JC, de la Rua ER, Martin F: Proliferative vitreoretinopathy: risk factors and pathobiology. Prog Retin Eye Res 21: 127–144, 2002 - PubMed
- U.K. Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AI050775/AI/NIAID NIH HHS/United States
- EY14104/EY/NEI NIH HHS/United States
- R01 HL086933/HL/NHLBI NIH HHS/United States
- P30 EY014104/EY/NEI NIH HHS/United States
- AI050775/AI/NIAID NIH HHS/United States
- HL086933/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous