Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1158/1940-6207.CAPR-08-0042.
Christine E McLaren, Daniel Pelot, Sharon Fujikawa-Brooks, Philip M Carpenter, Ernest Hawk, Gary Kelloff, Michael J Lawson, Jayashri Kidao, John McCracken, C Gregory Albers, Dennis J Ahnen, D Kim Turgeon, Steven Goldschmid, Peter Lance, Curt H Hagedorn, Daniel L Gillen, Eugene W Gerner
Affiliations
- PMID: 18841250
- PMCID: PMC2562024
- DOI: 10.1158/1940-6207.CAPR-08-0042
Randomized Controlled Trial
Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial
Frank L Meyskens Jr et al. Cancer Prev Res (Phila). 2008 Jun.
Abstract
Preclinical studies of chemoprevention drugs given in combination at low doses show remarkable efficacy in preventing adenomas with little additional toxicities, suggesting a strategy to improve risk to benefit ratios for preventing recurrent adenomas. Three hundred seventy-five patients with history of resected (> or =3 mm) adenomas were randomly assigned to receive oral difluoromethylornithine (DFMO) 500 mg and sulindac 150 mg once daily or matched placebos for 36 months, stratified by use of low-dose aspirin (81 mg) at baseline and clinical site. Follow-up colonoscopy was done 3 years after randomization or off-study. Colorectal adenoma recurrence was compared among the groups with log-binomial regression. Comparing the outcome in patients receiving placebos to those receiving active intervention, (a) the recurrence of one or more adenomas was 41.1% and 12.3% (risk ratio, 0.30; 95% confidence interval, 0.18-0.49; P < 0.001); (b) 8.5% had one or more advanced adenomas, compared with 0.7% of patients (risk ratio, 0.085; 95% confidence interval, 0.011-0.65; P < 0.001); and (c) 17 (13.2%) patients had multiple adenomas (>1) at the final colonoscopy, compared with 1 (0.7%; risk ratio, 0.055; 0.0074-0.41; P < 0.001). Serious adverse events (grade > or =3) occurred in 8.2% of patients in the placebo group, compared with 11% in the active intervention group (P = 0.35). There was no significant difference in the proportion of patients reporting hearing changes from baseline. Recurrent adenomatous polyps can be markedly reduced by a combination of low oral doses of DFMO and sulindac and with few side effects.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
MJ Lawson: Bristol-Myers Squibb Commercial Research Grant, Elan Pharmaceuticals Consultant. The other authors disclosed no potential conflicts of interest.
Figures
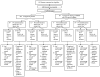
Fig. 1
Study schema.
Comment in
- Clinical prevention of recurrence of colorectal adenomas by the combination of difluoromethylornithine and sulindac: an important milestone.
Sporn MB, Hong WK. Sporn MB, et al. Cancer Prev Res (Phila). 2008 Jun;1(1):9-11. doi: 10.1158/1940-6207.CAPR-08-0049. Epub 2008 Apr 14. Cancer Prev Res (Phila). 2008. PMID: 19138930 No abstract available.
Similar articles
- Role of obesity in a randomized placebo-controlled trial of difluoromethylornithine (DFMO) + sulindac for the prevention of sporadic colorectal adenomas.
Zell JA, Lin BS, Madson N, McLaren CE, Gerner EW, Meyskens FL. Zell JA, et al. Cancer Causes Control. 2012 Oct;23(10):1739-44. doi: 10.1007/s10552-012-0051-6. Epub 2012 Aug 21. Cancer Causes Control. 2012. PMID: 22907422 Free PMC article. Clinical Trial. - Risk of cardiovascular events in a randomized placebo-controlled, double-blind trial of difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas.
Zell JA, Pelot D, Chen WP, McLaren CE, Gerner EW, Meyskens FL. Zell JA, et al. Cancer Prev Res (Phila). 2009 Mar;2(3):209-12. doi: 10.1158/1940-6207.CAPR-08-0203. Epub 2009 Mar 3. Cancer Prev Res (Phila). 2009. PMID: 19258540 Free PMC article. Clinical Trial. - Longitudinal assessment of air conduction audiograms in a phase III clinical trial of difluoromethylornithine and sulindac for prevention of sporadic colorectal adenomas.
McLaren CE, Fujikawa-Brooks S, Chen WP, Gillen DL, Pelot D, Gerner EW, Meyskens FL Jr. McLaren CE, et al. Cancer Prev Res (Phila). 2008 Dec;1(7):514-21. doi: 10.1158/1940-6207.CAPR-08-0074. Cancer Prev Res (Phila). 2008. PMID: 19139001 Free PMC article. Clinical Trial. - Polyamines as mediators of APC-dependent intestinal carcinogenesis and cancer chemoprevention.
Rial NS, Meyskens FL, Gerner EW. Rial NS, et al. Essays Biochem. 2009 Nov 4;46:111-24. doi: 10.1042/bse0460008. Essays Biochem. 2009. PMID: 20095973 Free PMC article. Review. - Nonsteroidal anti-inflammatory drugs and aspirin for the prevention of colorectal adenomas and cancer: a systematic review.
Asano TK, McLeod RS. Asano TK, et al. Dis Colon Rectum. 2004 May;47(5):665-73. doi: 10.1007/s10350-003-0111-9. Epub 2004 Apr 2. Dis Colon Rectum. 2004. PMID: 15054679 Review.
Cited by
- Distinct Local and Systemic Molecular Signatures in the Esophageal and Gastric Cancers: Possible Therapy Targets and Biomarkers for Gastric Cancer.
Bednarz-Misa I, Fortuna P, Diakowska D, Jamrozik N, Krzystek-Korpacka M. Bednarz-Misa I, et al. Int J Mol Sci. 2020 Jun 25;21(12):4509. doi: 10.3390/ijms21124509. Int J Mol Sci. 2020. PMID: 32630408 Free PMC article. - Role of dietary polyamines in a phase III clinical trial of difluoromethylornithine (DFMO) and sulindac for prevention of sporadic colorectal adenomas.
Raj KP, Zell JA, Rock CL, McLaren CE, Zoumas-Morse C, Gerner EW, Meyskens FL. Raj KP, et al. Br J Cancer. 2013 Feb 19;108(3):512-8. doi: 10.1038/bjc.2013.15. Epub 2013 Jan 22. Br J Cancer. 2013. PMID: 23340449 Free PMC article. - Chemoprevention of gastrointestinal cancer: the reality and the dream.
Chun KS, Kim EH, Lee S, Hahm KB. Chun KS, et al. Gut Liver. 2013 Mar;7(2):137-49. doi: 10.5009/gnl.2013.7.2.137. Epub 2013 Feb 7. Gut Liver. 2013. PMID: 23560148 Free PMC article. - The evolving role of nonsteroidal anti-inflammatory drugs in colon cancer prevention: a cause for optimism.
Rigas B, Tsioulias GJ. Rigas B, et al. J Pharmacol Exp Ther. 2015 Apr;353(1):2-8. doi: 10.1124/jpet.114.220806. J Pharmacol Exp Ther. 2015. PMID: 25589413 Free PMC article. Review. - Esophageal Squamous Cell Carcinoma Is Accompanied by Local and Systemic Changes in L-arginine/NO Pathway.
Bednarz-Misa I, Fortuna P, Fleszar MG, Lewandowski Ł, Diakowska D, Rosińczuk J, Krzystek-Korpacka M. Bednarz-Misa I, et al. Int J Mol Sci. 2020 Aug 30;21(17):6282. doi: 10.3390/ijms21176282. Int J Mol Sci. 2020. PMID: 32872669 Free PMC article.
References
- Minino AM, Heron MP, Murphy SL, Kochanek KD. Centers for Disease Control and Prevention National Center for Health Statistics National Vital Statistics S. Deaths: final data for 2004 Natl Vital Stat Rep. 2007;55:1–119. - PubMed
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
- Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–81. - PubMed
- Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127:1661–9. [see comment] - PubMed
- Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006;296:2815–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA059024/CA/NCI NIH HHS/United States
- CA72008/CA/NCI NIH HHS/United States
- P30 CA023074/CA/NCI NIH HHS/United States
- CA95060/CA/NCI NIH HHS/United States
- CA47396/CA/NCI NIH HHS/United States
- N01 CN075019/CN/NCI NIH HHS/United States
- CA88078/CA/NCI NIH HHS/United States
- N01-CN75019/CN/NCI NIH HHS/United States
- P50 CA095060/CA/NCI NIH HHS/United States
- P50 CA095060-05/CA/NCI NIH HHS/United States
- R01 CA123065/CA/NCI NIH HHS/United States
- P01 CA072008/CA/NCI NIH HHS/United States
- P30 CA023074-209010/CA/NCI NIH HHS/United States
- R01 CA123065-01A2/CA/NCI NIH HHS/United States
- CA63640/CA/NCI NIH HHS/United States
- P30 CA062203/CA/NCI NIH HHS/United States
- R01 CA088078/CA/NCI NIH HHS/United States
- R01 CA063640/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical