Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex - PubMed (original) (raw)
Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex
Leda Dimou et al. J Neurosci. 2008.
Abstract
Despite their abundance, still little is known about the rather frequent, constantly proliferating progenitors spread throughout the adult mouse brain parenchyma. The majority of these progenitors express the basic-helix-loop-helix transcription factor Olig2, and their number further increases after injury. Here, we examine the progeny of this progenitor population by genetic fate mapping using tamoxifen-inducible Cre-recombination in the Olig2 locus to turn on permanent reporter gene expression in the adult brain. Consistent with Olig2 expression in proliferating NG2(+) progenitors, most reporter(+) cells seen shortly after initiating recombination at adult stages incorporated BrdU and contained the proteoglycan NG2 in both the gray (GM) and the white matter (WM) of the cerebral cortex. However, at longer time points after induction, we observed profound differences in the identity of reporter(+) cells in the WM and GM. Whereas most of the Olig2(+) progenitors had generated mature, myelinating oligodendrocytes in the WM, hardly any reporter(+) cells showing mature oligodendrocyte characteristics were detectable even up to 6 months after recombination in the GM. In the GM, most reporter(+) cells remained NG2(+), even after injury, but stopped proliferating rather soon after recombination. Thus, our results demonstrate the continuous generation of mature, myelinating oligodendrocytes in the WM, whereas cells in the GM generated mostly postmitotic NG2(+) glia.
Figures
Figure 1.
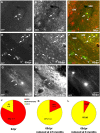
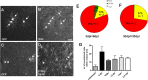
Identification of reporter+ cells in the WM. A–I, Micrographs of examples of GFP+ cells 8 (A–C) or 65 (D–I) dpr double-stained for cell type-specific antigens. The pies in J and K depict the quantitative cell type analysis among reporter+ cells 8 (J) or 65 (K) dpr recombined in 2.5-month-old animals. L, Cell type analysis at 65 dpr in mice recombined at 6 months of age. Arrows point to double-stained cells; arrowheads point to single positive cells. Scale bars, 20 μm.
Figure 2.
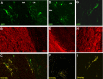
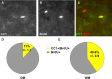
A–I, Confocal microscope images showing the colabeling of reporter+ cells and myelin sheaths with the myelin markers MAG (A–C), MOG (D–F), and MBP (G–I) in the white matter (corpus callosum) at 65 dpr. C, F, I, Colocalizing pixels are in yellow.
Figure 3.
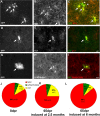
Identification of reporter+ cells in the GM. A–I, Micrographs of GFP+ cells at 8 dpr. Arrows point to colabeled cells, arrowheads to reporter+ cells negative for the cell type-specific antigen. Pies in J and K depict the quantitative cell type analysis of reporter+ cells (J, 8 dpr; K, 65 dpr) recombined in 2.5-month-old animals. L, Cell type analysis at 65 dpr in mice recombined at 6 months of age. Scale bars, 20 μm.
Figure 4.
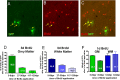
A–C, Identification of proliferating reporter+ cells at 8 dpr. D–F, Graphs in D and E show the proportion of recombined cells that are proliferating at 8, 30, and 120 dpr in the gray (D) and white matter (E) and at 6 and 9 dpr in both areas (F). BrdU was applied for a period of 3 d (D, E) or 1 d (F) in the drinking water, directly before analysis of the animals.
Figure 5.
Identification of reporter+ cells after stab wound injury in the GM. A–D, Micrographs of GFP+ cells in the lesion site at 3 dpl (8 dpr) double-stained for cell type-specific antigens as indicated in the panels. Pies in E and F depict the quantitative analysis of reporter+ cells with regard to cell identity (E, 3 dpl; F, 30 dpl). Quantification of absolute numbers of recombined cells at different time points after the stab wound within the lesion area localized 150 μm away from the lesion track is shown in G. Scale bar, 20 μm.
Figure 6.
A–C, Colabeling of BrdU with CC1 in the GM. Pies in D and E show the proportion of BrdU+ cells colabeling with CC1 (yellow) in the GM (D) and in the WM (E) after application of BrdU in adult wild-type mice.
Similar articles
- Olig2/Plp-positive progenitor cells give rise to Bergmann glia in the cerebellum.
Chung SH, Guo F, Jiang P, Pleasure DE, Deng W. Chung SH, et al. Cell Death Dis. 2013 Mar 14;4(3):e546. doi: 10.1038/cddis.2013.74. Cell Death Dis. 2013. PMID: 23492777 Free PMC article. - NG2-proteoglycan-dependent contributions of oligodendrocyte progenitors and myeloid cells to myelin damage and repair.
Kucharova K, Stallcup WB. Kucharova K, et al. J Neuroinflammation. 2015 Sep 4;12:161. doi: 10.1186/s12974-015-0385-6. J Neuroinflammation. 2015. PMID: 26338007 Free PMC article. - Cytology and lineage of NG2-positive glia.
Berry M, Hubbard P, Butt AM. Berry M, et al. J Neurocytol. 2002 Jul-Aug;31(6-7):457-67. doi: 10.1023/a:1025735513560. J Neurocytol. 2002. PMID: 14501216 Review. - NG2-expressing cells as oligodendrocyte progenitors in the normal and demyelinated adult central nervous system.
Polito A, Reynolds R. Polito A, et al. J Anat. 2005 Dec;207(6):707-16. doi: 10.1111/j.1469-7580.2005.00454.x. J Anat. 2005. PMID: 16367798 Free PMC article. Review.
Cited by
- Aging and senescent fates of oligodendrocyte precursor cells in the mouse brain.
Gomez PT, Carver CM, Rodriguez SL, Wang L, Zhang X, Schafer MJ. Gomez PT, et al. NPJ Aging. 2024 Oct 22;10(1):47. doi: 10.1038/s41514-024-00176-y. NPJ Aging. 2024. PMID: 39438481 Free PMC article. - Glial progenitor cells of the adult human white and grey matter are contextually distinct.
Osorio MJ, Mariani JN, Zou L, Schanz SJ, Heffernan K, Cornwell A, Goldman SA. Osorio MJ, et al. Glia. 2023 Mar;71(3):524-540. doi: 10.1002/glia.24291. Epub 2022 Nov 5. Glia. 2023. PMID: 36334067 Free PMC article. - Clock Genes in Glia Cells: A Rhythmic History.
Chi-Castañeda D, Ortega A. Chi-Castañeda D, et al. ASN Neuro. 2016 Sep 25;8(5):1759091416670766. doi: 10.1177/1759091416670766. Print 2016 Oct. ASN Neuro. 2016. PMID: 27666286 Free PMC article. Review. - In vivo imaging reveals mature Oligodendrocyte division in adult Zebrafish.
Zou S, Hu B. Zou S, et al. Cell Regen. 2021 Jun 2;10(1):16. doi: 10.1186/s13619-021-00079-3. Cell Regen. 2021. PMID: 34075520 Free PMC article. - Oligodendrocyte degeneration and recovery after focal cerebral ischemia.
McIver SR, Muccigrosso M, Gonzales ER, Lee JM, Roberts MS, Sands MS, Goldberg MP. McIver SR, et al. Neuroscience. 2010 Sep 1;169(3):1364-75. doi: 10.1016/j.neuroscience.2010.04.070. Epub 2010 May 31. Neuroscience. 2010. PMID: 20621643 Free PMC article.
References
- Bartsch U, Kirchhoff F, Schachner M. Immunohistological localization of the adhesion molecules L1, N-CAM, and MAG in the developing and adult optic nerve of mice. J Comp Neurol. 1989;284:451–462. - PubMed
- Battiste J, Helms AW, Kim EJ, Savage TK, Lagace DC, Mandyam CD, Eisch AJ, Miyoshi G, Johnson JE. Ascl1 defines sequentially generated lineage-restricted neuronal and oligodendrocyte precursor cells in the spinal cord. Development. 2007;134:285–293. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases