Amyloid beta-protein assembly and Alzheimer disease - PubMed (original) (raw)
Review
Amyloid beta-protein assembly and Alzheimer disease
Robin Roychaudhuri et al. J Biol Chem. 2009.
No abstract available
Figures
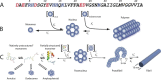
FIGURE 1.
A_β_ assembly. A, the sequence of A_β_42 is shown in one-letter amino acid code. The side chain charge at neutral pH is color-coded (red, negative; blue, positive). B, nucleation-dependent polymerization, reflecting the unfavorable self-association (rate constant kn+≪kn−) of X natively folded monomers (in this case, six total) to form a fibril nucleus and the favorable addition (ke+≫ke−) of a large indeterminate number of monomers to the nucleus (nascent fibril) during fibril elongation. C, A_β_self-assembly. A_β_belongs to the class of “natively disordered” proteins, existing in the monomer state as an equilibrium mixture of many conformers. On-pathway assembly requires the formation of a partially folded monomer that self-associates to form a nucleus for fibril elongation, a paranucleus (in this case, containing six monomers). Nucleation of monomer folding is a process distinct from fibril nucleation (50). Fibril nucleation is unfavorable kinetically (k2+≪k2−), which explains the lag phase of fibrillogenesis experiments, a period during which no fibril formation is apparent. Paranuclei self-associate readily (k3+≫k3−) to form protofibrils, which are relatively narrow (∼5 nm), short (<150 nm), flexible structures. These protofibrils comprise a significant but finite number (_X_) of paranuclei. Maturation of protofibrils through a process that is kinetically favorable (k4+>k4−) yields classical amyloid-type fibrils (∼10-nm diameter, indeterminate (but often >1 _μ_m) length). Other assembly pathways produce annular pore-like structures, globular dodecameric (and higher order) structures, and amylospheroids. Annuli and amylospheroids appear to be off-pathway assemblies.
Similar articles
- Polymorphism in Alzheimer Abeta amyloid organization reflects conformational selection in a rugged energy landscape.
Miller Y, Ma B, Nussinov R. Miller Y, et al. Chem Rev. 2010 Aug 11;110(8):4820-38. doi: 10.1021/cr900377t. Chem Rev. 2010. PMID: 20402519 Free PMC article. Review. No abstract available. - Alzheimer amyloid hypothesis lives on.
Mullard A. Mullard A. Nat Rev Drug Discov. 2016 Dec 29;16(1):3-5. doi: 10.1038/nrd.2016.281. Nat Rev Drug Discov. 2016. PMID: 28031570 No abstract available. - Explaining the cause of the amyloid burden in Alzheimer disease.
Rosenberg RN. Rosenberg RN. Arch Neurol. 2002 Sep;59(9):1367-8. doi: 10.1001/archneur.59.9.1367. Arch Neurol. 2002. PMID: 12223021 No abstract available. - β-adrenergic receptor, amyloid β-peptide, and Alzheimer's disease.
Wang D, Xiang YK. Wang D, et al. Curr Top Membr. 2011;67:205-28. doi: 10.1016/B978-0-12-384921-2.00010-0. Curr Top Membr. 2011. PMID: 21771492 Review. No abstract available. - ADDLs & protofibrils--the missing links?
Klein WL. Klein WL. Neurobiol Aging. 2002 Mar-Apr;23(2):231-5. doi: 10.1016/s0197-4580(01)00312-8. Neurobiol Aging. 2002. PMID: 11804707 No abstract available.
Cited by
- Dynamics of metastable β-hairpin structures in the folding nucleus of amyloid β-protein.
Cruz L, Rao JS, Teplow DB, Urbanc B. Cruz L, et al. J Phys Chem B. 2012 Jun 7;116(22):6311-25. doi: 10.1021/jp301619v. Epub 2012 May 24. J Phys Chem B. 2012. PMID: 22587454 Free PMC article. - Z-Phe-Ala-diazomethylketone (PADK) disrupts and remodels early oligomer states of the Alzheimer disease Aβ42 protein.
Zheng X, Gessel MM, Wisniewski ML, Viswanathan K, Wright DL, Bahr BA, Bowers MT. Zheng X, et al. J Biol Chem. 2012 Feb 24;287(9):6084-8. doi: 10.1074/jbc.C111.328575. Epub 2012 Jan 17. J Biol Chem. 2012. PMID: 22253440 Free PMC article. - Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding.
Ono K, Li L, Takamura Y, Yoshiike Y, Zhu L, Han F, Mao X, Ikeda T, Takasaki J, Nishijo H, Takashima A, Teplow DB, Zagorski MG, Yamada M. Ono K, et al. J Biol Chem. 2012 Apr 27;287(18):14631-43. doi: 10.1074/jbc.M111.325456. Epub 2012 Mar 5. J Biol Chem. 2012. PMID: 22393064 Free PMC article. - Soluble prion protein inhibits amyloid-β (Aβ) fibrillization and toxicity.
Nieznanski K, Choi JK, Chen S, Surewicz K, Surewicz WK. Nieznanski K, et al. J Biol Chem. 2012 Sep 28;287(40):33104-8. doi: 10.1074/jbc.C112.400614. Epub 2012 Aug 22. J Biol Chem. 2012. PMID: 22915585 Free PMC article. - C-terminal turn stability determines assembly differences between Aβ40 and Aβ42.
Roychaudhuri R, Yang M, Deshpande A, Cole GM, Frautschy S, Lomakin A, Benedek GB, Teplow DB. Roychaudhuri R, et al. J Mol Biol. 2013 Jan 23;425(2):292-308. doi: 10.1016/j.jmb.2012.11.006. Epub 2012 Nov 12. J Mol Biol. 2013. PMID: 23154165 Free PMC article.
References
- Kirkitadze MD, Bitan G, Teplow DB. J Neurosci Res. 2002;69:567–577. - PubMed
- Nelson R, Eisenberg D. Curr Opin Struct Biol. 2006;16:260–265. - PubMed
- Teplow DB. Methods Enzymol. 2006;413:20–33. - PubMed
- Finder VH, Glockshuber R. Neurodegener Dis. 2007;4:13–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical