Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency - PubMed (original) (raw)
Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency
Jared E Knickelbein et al. Science. 2008.
Abstract
Reactivation of herpes simplex virus type 1 (HSV-1) from neuronal latency is a common and potentially devastating cause of disease worldwide. CD8+ T cells can completely inhibit HSV reactivation in mice, with interferon-gamma affording a portion of this protection. We found that CD8+ T cell lytic granules are also required for the maintenance of neuronal latency both in vivo and in ex vivo ganglia cultures and that their directed release to the junction with neurons in latently infected ganglia did not induce neuronal apoptosis. Here, we describe a nonlethal mechanism of viral inactivation in which the lytic granule component, granzyme B, degrades the HSV-1 immediate early protein, ICP4, which is essential for further viral gene expression.
Figures
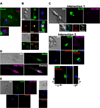
Figure 1. CD8+ T cells release lytic granules toward neurons within HSV-1 latently infected ganglia without activating neuronal caspases
(A) In situ confocal images of intact latently infected WT TG stained with antibodies to CD8α, GrB, and NeuN (neuronal nucleus). Top: CD8+ T cell with GrB polarized to the junction with a NeuN+ neuron. Bottom: Most CD8+ T cells have GrB dispersed throughout the cell. (B) Imaging of ex vivo cultures of latently infected WT TG. Top: CD8+ T cell in contact with two neurons polarizes TCR and GrB toward lower neuron only. Bottom: CD8+ T cells not contacting targets show no TCR or GrB polarization. (C–E) Imaging of ex vivo latently infected WT TG cultured 24–48 hrs with WT gB-CD8, which prevent reactivation from latency. (C) Two representative interactions between NeuN+ neurons and CD8+ T cells with CD107a polarized toward the junction with a neuron lacking activated caspases (VAD-FMK−). Interaction 2, bottom panel: the plane demarcated by the line between the cells (left) is shown en face (right) demonstrating an apparent secretory domain of an immunological synapse. (D) 10% ethanol induced neuronal caspase activation. (E) CD8+ T cells contacting NeuN− non-infected fibroblast-like cells (left) or not contacting cells (right) lack surface CD107a expression. Data are representative of 11 TG from three separate experiments.
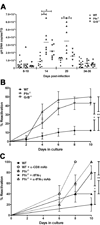
Figure 2. Pfn and GrB are required to maintain HSV-1 neuronal latency in vivo and in ex vivo ganglia cultures
(A) DNA was extracted from individual TG at designated times and HSV-1 genome copies was determined by quantitative real-time PCR (horizontal bar = mean). Data pooled from at least two independent experiments per time point. * p≤0.05 as calculated by ANOVA with Bonferroni post-test. (B) 34–41 dpi TG were dispersed and cultured at one-fifth TG equivalents per well. HSV-1 reactivation was indicated by the presence of infectious virus in serially sampled supernatants as assessed by plaque assay. Pooled data from three independent experiments presented as mean ± SEM. * p=0.0009; ** p=0.0002 as calculated by survival curve analysis (Log-rank test). (C) 14 dpi TG were dispersed and cultured at 1 TG per well in medium alone or in medium supplemented with 1000 U/ml recombinant IFN-γ (rIFN-γ), 100 μg/ml anti-CD8α monoclonal antibody (α-CD8α mAb), or 20 μg/ml anti-IFN-γ monoclonal antibody (α-IFN-γ mAb). Reactivation was assessed, analyzed, and presented as in (B). n=10 TG/condition. Data representative of two independent experiments. * p=0.0108; ** p=0.0049.
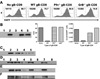
Figure 3. GrB cleaves the essential HSV-1 IE protein ICP4
(A&B) B6WT3 fibroblasts were infected with recombinant HSV-1 expressing EGFP from the ICP0 promoter for 1 hr, washed, and exposed to no, WT, Pfn−/−, or GrB−/− gB-CD8 for 5 hrs. (A) Histograms from flow analysis of recovered cells show total fibroblasts (CD8−) recovered from cultures (top left) and percent infected (EGFP+; top right). (B) Lysates of recovered cells were subjected to Western blot for ICP4 (Lane 1: Noninfected; Lanes 2–5: Infected with No, WT, Pfn−/−, or GrB−/− gB-CD8, respectively). Bar graphs show optical density (O.D.) readings that were not adjusted (middle) or adjusted for the number of infected fibroblasts recovered from each culture (right). (C) Lysates from 293T cells that were either non-transfected (lane 1) or transfected with an ICP4-expressing plasmid (lanes 2–5) and exposed to different concentrations of recombinant GrB (lanes 1–5: 0, 0, 25, 50, 100 nM GrB, respectively) for 1 hr at 37° C. Lysates from 293T cells transfected with an ICP4-expressing plasmid (D), lysates from B6WT3 cells infected with HSV-1 (E), or ICP4 immunoprecipitated from HSV-1-infected fibroblasts (F) were exposed to 0 (lanes 1–3, 5, 7) or 100 nM GrB (lanes 4, 6, 8) for varying times at 37° C (lanes 1–2: 0 hr; 3–4: 1 hr; 5–6: 2 hrs; 7–8: 3 hrs). Lane 1 contains non-transfected 293T cell lysate (D) or noninfected fibroblast lysate (E).
Similar articles
- Dok-1 and Dok-2 Are Required To Maintain Herpes Simplex Virus 1-Specific CD8+ T Cells in a Murine Model of Ocular Infection.
Lahmidi S, Yousefi M, Dridi S, Duplay P, Pearson A. Lahmidi S, et al. J Virol. 2017 Jul 12;91(15):e02297-16. doi: 10.1128/JVI.02297-16. Print 2017 Aug 1. J Virol. 2017. PMID: 28490594 Free PMC article. - CD8+ T Cells Play a Bystander Role in Mice Latently Infected with Herpes Simplex Virus 1.
Mott KR, Gate D, Matundan HH, Ghiasi YN, Town T, Ghiasi H. Mott KR, et al. J Virol. 2016 Apr 29;90(10):5059-5067. doi: 10.1128/JVI.00255-16. Print 2016 May 15. J Virol. 2016. PMID: 26962220 Free PMC article. - Medroxyprogesterone acetate inhibits CD8+ T cell viral-specific effector function and induces herpes simplex virus type 1 reactivation.
Cherpes TL, Busch JL, Sheridan BS, Harvey SA, Hendricks RL. Cherpes TL, et al. J Immunol. 2008 Jul 15;181(2):969-75. doi: 10.4049/jimmunol.181.2.969. J Immunol. 2008. PMID: 18606648 Free PMC article. - Control of HSV-1 latency in human trigeminal ganglia--current overview.
Held K, Derfuss T. Held K, et al. J Neurovirol. 2011 Dec;17(6):518-27. doi: 10.1007/s13365-011-0063-0. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139603 Review. - CD8+ T cells patrol HSV-1-infected trigeminal ganglia and prevent viral reactivation.
St Leger AJ, Hendricks RL. St Leger AJ, et al. J Neurovirol. 2011 Dec;17(6):528-34. doi: 10.1007/s13365-011-0062-1. Epub 2011 Dec 8. J Neurovirol. 2011. PMID: 22161682 Review.
Cited by
- Orchestration of antiviral responses within the infected central nervous system.
Pavlou A, Mulenge F, Gern OL, Busker LM, Greimel E, Waltl I, Kalinke U. Pavlou A, et al. Cell Mol Immunol. 2024 Sep;21(9):943-958. doi: 10.1038/s41423-024-01181-7. Epub 2024 Jul 12. Cell Mol Immunol. 2024. PMID: 38997413 Free PMC article. Review. - Herpes Simplex Virus Type 1 Interactions with the Interferon System.
Danastas K, Miranda-Saksena M, Cunningham AL. Danastas K, et al. Int J Mol Sci. 2020 Jul 21;21(14):5150. doi: 10.3390/ijms21145150. Int J Mol Sci. 2020. PMID: 32708188 Free PMC article. Review. - Pan-HSV-2 IgG antibody in vaccinated mice and guinea pigs correlates with protection against herpes simplex virus 2.
Halford WP, Geltz J, Gershburg E. Halford WP, et al. PLoS One. 2013 Jun 6;8(6):e65523. doi: 10.1371/journal.pone.0065523. Print 2013. PLoS One. 2013. PMID: 23755244 Free PMC article. - Immune modulation during latent herpesvirus infection.
White DW, Suzanne Beard R, Barton ES. White DW, et al. Immunol Rev. 2012 Jan;245(1):189-208. doi: 10.1111/j.1600-065X.2011.01074.x. Immunol Rev. 2012. PMID: 22168421 Free PMC article. Review. - The immunobiology of corneal HSV-1 infection and herpetic stromal keratitis.
Antony F, Kinha D, Nowińska A, Rouse BT, Suryawanshi A. Antony F, et al. Clin Microbiol Rev. 2024 Sep 12;37(3):e0000624. doi: 10.1128/cmr.00006-24. Epub 2024 Jul 30. Clin Microbiol Rev. 2024. PMID: 39078136 Review.
References
- Hufner K, et al. J Neuropathol Exp Neurol. 2006;65:1022. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01EY05945/EY/NEI NIH HHS/United States
- P30EY08098/EY/NEI NIH HHS/United States
- F30NS061471/NS/NINDS NIH HHS/United States
- R01EY015291/EY/NEI NIH HHS/United States
- F30 NS061471-01/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials