Resistance exercise increases human skeletal muscle AS160/TBC1D4 phosphorylation in association with enhanced leg glucose uptake during postexercise recovery - PubMed (original) (raw)
Resistance exercise increases human skeletal muscle AS160/TBC1D4 phosphorylation in association with enhanced leg glucose uptake during postexercise recovery
Hans C Dreyer et al. J Appl Physiol (1985). 2008 Dec.
Abstract
Akt substrate of 160 kDa (AS160/TBC1D4) is associated with insulin and contraction-mediated glucose uptake. Human skeletal muscle AS160 phosphorylation is increased during aerobic exercise but not immediately following resistance exercise. It is not known whether AS160 phosphorylation is altered during recovery from resistance exercise. Therefore, we hypothesized that muscle AS160/TBC1D4 phosphorylation and glucose uptake across the leg would be increased during recovery following resistance exercise. We studied 9 male subjects before, during, and for 2 h of postexercise recovery. We utilized femoral catheterizations and muscle biopsies in combination with indirect calorimetry and immunoblotting to determine whole body glucose and fat oxidation, leg glucose uptake, muscle AMPKalpha2 activity, and the phosphorylation of muscle Akt and AS160/TBC1D4. Glucose oxidation was reduced while fat oxidation increased ( approximately 35%) during postexercise recovery (P <or= 0.05). Glucose uptake increased during exercise and postexercise recovery (P <or= 0.05). Akt phosphorylation was increased at 1 h and AMPKalpha2 activity increased at 2 h postexercise (P <or= 0.05). Phospho(Ser/Thr)-Akt substrate (PAS) phosphorylation (often used as a marker for AS160) was unchanged immediately postexercise and increased at 1 h (P <or= 0.05) and 2 h postexercise (P = 0.07). The PAS antibody is not always specific for AS160/TBC1D4 and can detect proteins at a similar molecular weight. Therefore, we immunoprecipitated AS160/TBC1D4 and then blotted with the PAS antibody, which confirmed that PAS phosphorylation is occurring on AS160/TBC1D4. There was also a positive correlation between PAS phosphorylation and leg glucose uptake during recovery (P < 0.05). We conclude that resistance exercise increases AS160/TBC1D4 phosphorylation in association with an increase in leg glucose uptake during postexercise recovery.
Figures
Fig. 1.
Study design and timeline in hours. Schematic displaying the study design used to measure the effect of resistance exercise on the regulation of whole body fat and glucose oxidation, glucose uptake, and cell signaling in male subjects. The study design consisted of a baseline, exercise, 1-h postexercise, and 2-h postexercise period. Continuous breath sampling was obtained with a Sensor Medics Vmax series metabolic cart. Indocyanine green (ICG) was infused to measure blood flow during each period. Blood samples were collected to measure plasma glucose and insulin and blood flow and to calculate glucose uptake. Muscle biopsies were used to measure muscle cell signaling, specifically components upstream and partially responsible for the activation of Akt substrate of 160 kDa (AS160).
Fig. 2.
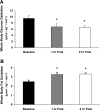
Whole body glucose oxidation and postexercise fat oxidation. Breath samples were continuously obtained with the Vmax series metabolic cart during each period of the study. Whole body glucose and fat oxidation during exercise are not reported due to a respiratory quotient (RQ) being greater than 1. A: whole body glucose oxidation. B: whole body fat oxidation. Data are expressed as means ± SE. 1 h Post, 1 h postexercise; 2 h Post, 2 h postexercise. *P ≤ 0.05 vs. baseline.
Fig. 3.
Blood insulin and glucose concentrations. Blood samples were obtained 4 times at regular intervals (approximately every 10 min.) during each period of the study: baseline, exercise, 1 h postexercise, and 2 h postexercise. Venous insulin and glucose concentrations. Data are expressed as means ± SE. *P ≤ 0.05 vs. baseline.
Fig. 4.
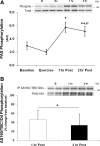
ACC phosphorylation. Data are from each biopsy taken at baseline, immediately following resistance exercise (Exercise), 1 h following exercise, and 2 h following resistance exercise. Data are expressed as means ± SE. *P ≤ 0.05 vs. baseline (n = 7). Insets: representative blots for each time point: baseline (B), exercise (Ex), 1 h postexercise (1 h), and 2 h postexercise (2 h).
Fig. 5.
Phospho(Ser/Thr)-Akt substrate (PAS) phosphorylation (A) and immunoprecipitation of AS160/TBC1D4 (B). A: data are from each biopsy taken at baseline, immediately following resistance exercise, 1 h following exercise, and 2 h following resistance exercise for PAS160 phosphorylation. *P ≤ 0.05 vs. baseline; n = 7. B: AS160/TBC1D4 was first immunoprecipitated and then blotted with the PAS antibody. Data are expressed as means ± SE. *P ≤ 0.05 time effect; n = 6. Insets: representative blots for each time point [baseline (B), exercise (Ex), 1 h postexercise (1 h), and 2 h postexercise (2 h)].
Fig. 6.
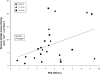
Correlation of PAS phosphorylation and glucose uptake across the leg. Data points from baseline and 1 h and 2 h post-resistance exercise are included in the Pearson product-moment correlation (24 data points). PAS phosphorylation was positively correlated with glucose uptake across the leg (R = 0.41; P ≤ 0.05).
Similar articles
- Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle.
Cartee GD. Cartee GD. Diabetologia. 2015 Jan;58(1):19-30. doi: 10.1007/s00125-014-3395-5. Epub 2014 Oct 4. Diabetologia. 2015. PMID: 25280670 Free PMC article. Review. - Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle.
Funai K, Schweitzer GG, Sharma N, Kanzaki M, Cartee GD. Funai K, et al. Am J Physiol Endocrinol Metab. 2009 Jul;297(1):E242-51. doi: 10.1152/ajpendo.00194.2009. Epub 2009 May 12. Am J Physiol Endocrinol Metab. 2009. PMID: 19435856 Free PMC article. - Exercise increases TBC1D1 phosphorylation in human skeletal muscle.
Jessen N, An D, Lihn AS, Nygren J, Hirshman MF, Thorell A, Goodyear LJ. Jessen N, et al. Am J Physiol Endocrinol Metab. 2011 Jul;301(1):E164-71. doi: 10.1152/ajpendo.00042.2011. Epub 2011 Apr 19. Am J Physiol Endocrinol Metab. 2011. PMID: 21505148 Free PMC article. Clinical Trial. - Postexercise improvement in glucose uptake occurs concomitant with greater γ3-AMPK activation and AS160 phosphorylation in rat skeletal muscle.
Wang H, Arias EB, Pataky MW, Goodyear LJ, Cartee GD. Wang H, et al. Am J Physiol Endocrinol Metab. 2018 Nov 1;315(5):E859-E871. doi: 10.1152/ajpendo.00020.2018. Epub 2018 Aug 21. Am J Physiol Endocrinol Metab. 2018. PMID: 30130149 Free PMC article. - Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport.
Cartee GD, Wojtaszewski JF. Cartee GD, et al. Appl Physiol Nutr Metab. 2007 Jun;32(3):557-66. doi: 10.1139/H07-026. Appl Physiol Nutr Metab. 2007. PMID: 17510697 Review.
Cited by
- A Review of Rehabilitation Benefits of Exercise Training Combined with Nutrition Supplement for Improving Protein Synthesis and Skeletal Muscle Strength in Patients with Cerebral Stroke.
Liu S, Liu H, Yang L, Wang K, Chen N, Zhang T, Luo J. Liu S, et al. Nutrients. 2022 Nov 24;14(23):4995. doi: 10.3390/nu14234995. Nutrients. 2022. PMID: 36501025 Free PMC article. Review. - Endocrine responses following exhaustive strength exercise with and without the use of protein and protein-carbohydrate supplements.
Wilk M, Michalczyk M, Gołaś A, Krzysztofik M, Maszczyk A, Zając A. Wilk M, et al. Biol Sport. 2018 Dec;35(4):399-405. doi: 10.5114/biolsport.2018.75754. Epub 2018 Nov 13. Biol Sport. 2018. PMID: 30765926 Free PMC article. - Exercise type and volume alter signaling pathways regulating skeletal muscle glucose uptake and protein synthesis.
Ahtiainen JP, Walker S, Silvennoinen M, Kyröläinen H, Nindl BC, Häkkinen K, Nyman K, Selänne H, Hulmi JJ. Ahtiainen JP, et al. Eur J Appl Physiol. 2015 Sep;115(9):1835-45. doi: 10.1007/s00421-015-3155-3. Epub 2015 Apr 10. Eur J Appl Physiol. 2015. PMID: 25861013 Clinical Trial. - Roles of TBC1D1 and TBC1D4 in insulin- and exercise-stimulated glucose transport of skeletal muscle.
Cartee GD. Cartee GD. Diabetologia. 2015 Jan;58(1):19-30. doi: 10.1007/s00125-014-3395-5. Epub 2014 Oct 4. Diabetologia. 2015. PMID: 25280670 Free PMC article. Review. - Effects of weight management by exercise modes on markers of subclinical atherosclerosis and cardiometabolic profile among women with abdominal obesity: a randomized controlled trial.
Choo J, Lee J, Cho JH, Burke LE, Sekikawa A, Jae SY. Choo J, et al. BMC Cardiovasc Disord. 2014 Jul 10;14:82. doi: 10.1186/1471-2261-14-82. BMC Cardiovasc Disord. 2014. PMID: 25011384 Free PMC article. Clinical Trial.
References
- Arias EB, Kim J, Funai K, Cartee GD. Prior exercise increases phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle. Am J Physiol Endocrinol Metab 292: E1191–E1200, 2007. - PubMed
- Bouzakri K, Karlsson HK, Vestergaard H, Madsbad S, Christiansen E, Zierath JR. IRS-1 serine phosphorylation and insulin resistance in skeletal muscle from pancreas transplant recipients. Diabetes 55: 785–791, 2006. - PubMed
- Bruss MD, Arias EB, Lienhard GE, Cartee GD. Increased phosphorylation of Akt substrate of 160 kDa (AS160) in rat skeletal muscle in response to insulin or contractile activity. Diabetes 54: 41–50, 2005. - PubMed
- Cartee GD, Wojtaszewski JF. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl Physiol Nutr Metab 32: 557–566, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-AR-049877/AR/NIAMS NIH HHS/United States
- S10-RR-16650/RR/NCRR NIH HHS/United States
- M01-RR-00073/RR/NCRR NIH HHS/United States
- K12 HD055929/HD/NICHD NIH HHS/United States
- R01 AR049877/AR/NIAMS NIH HHS/United States
- P30-AG-024832/AG/NIA NIH HHS/United States
- P30 AG024832/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical