Autophagy regulates selective HMGB1 release in tumor cells that are destined to die - PubMed (original) (raw)
Autophagy regulates selective HMGB1 release in tumor cells that are destined to die
J Thorburn et al. Cell Death Differ. 2009 Jan.
Abstract
Macroautophagy (hereafter referred to as autophagy) can increase or decrease the amount of cell death in response to various stimuli. To test whether autophagy also controls the characteristics associated with dying cells, we studied tumor cell killing by epidermal growth factor receptor-targeted diphtheria toxin (DT-EGF). DT-EGF kills epithelial and glioblastoma tumor cells with similar efficiency but by different mechanisms that depend on whether the cells activate autophagy when treated with the drug. Dying cells in which autophagy is induced selectively release the immune modulator high-mobility group B1 (HMGB1) without causing lysis of the cell membrane and classical necrosis. Conversely, cells that are killed by DT-EGF where autophagy is blocked, activate caspases but retain HMGB1. These data suggest that it may be feasible to manipulate the immunogenicity of dying cells by increasing or decreasing autophagy.
Figures
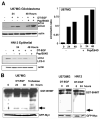
Figure 1. DT-EGF kills glioblastoma and epithelial tumor cells by different mechanisms
Panel A, quantification of clonogenic growth assay after treatment of HeLa, HN12, U87MG and U373MG cells for 24 hours with increasing doses of DT-EGF. All the cell lines die with similar dose responses (mean +/- SD from three experiments). Panel B shows frames from time-lapse movies of DT-EGF-treated HeLa cells and U87MG cells, showing that the morphological characteristics of the dying cells differs with only the epithelial cells displaying characteristics of apoptosis. Panel C, western analysis of PARP cleavage after 24 hours of DT-EGF treatment. Only the epithelial cells contain caspase-cleaved PARP.
Figure 2. DT-EGF induces autophagy in glioblastoma cells
Panel A, 2D-DIGE of lexatumumab or DT-EGF-treated U87MG cells compared with untreated controls. Lexatumumab treatment causes many changes to the proteome whereas DT-EGF treatment shows few changes. The boxed area identifies a post-translational change involving phosphorylation of eEF2 that is induced by DT-EGF treatment. Panel B, U87MG cells stably expressing GFP-LC3 were treated with DT-EGF or trehalose after transfection of siRNAs as indicated. Aggregation of LC3 was monitored by fluorescence microscopy after 24 hours and quantitated (Panel C) by counting the percentage of cells with > 10 GFP-labeled dots, data shown is the mean +/- SD from two experiments. Both DT-EGF and trehalose induce LC3 aggregation that is abolished by knockdown of autophagy regulators; only DT-EGF-induced aggregation is blocked by EGFR knockdown.
Figure 3
Panel A, western analysis of LC3 processing in the presence or absence of lysosomal protease inhibitors pepstatin A and E64D. DT-EGF causes increased LC3-II, which was further stimulated by treatment with pepstatin and E64D in the glioblastoma cells but not in the HN12 epithelial cells. The histogram shows quantitation of the ratio of LC3-II compared to actin for U87MG cells. Panel B, processing of the autophagy cargo protein betaine homocysteine methyltransferase (BHMT). The arrow indicates the processed form of the protein that is produced in autophagolysosomes. U87MG and U373MG glioblastoma cells display DT-EGF-induced BHMT processing, HN12 epithelial cells do not. GFP-Myc indicates the loading control, which was expressed from the GST-BHMT by an IRES sequence (26).
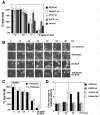
Figure 4. Autophagy protects against DT-EGF-induced death
Panel A, clonogenic survival assay in U87MG cells after siRNA knockdown as indicated and treatment with increasing doses of DT-EGF, data represents the mean +/- SD of four experiments each performed in triplicate. Knockdown of autophagy regulators increases DT-EGF-induced cell death. Panel B, frames from a time-lapse microscopy experiment of U87MG cells treated with DT-EGF with or without 24 hours pre-treatment with trehalose to further increase autophagy; increased autophagy delays DT-EGF-induced death. Panel C, clonogenic survival assays in U87MG cells treated with DT-EGF in the presence or absence of trehalose to increase autophagy (mean +/- SD from three experiments). Increased autophagy inhibits DT-EGF-induced death. Panel D, caspase 3/7 activity in DT-EGF-treated U87MG cells after siRNA treatment (mean +/- SD from three replicates). Control and EGFR siRNA, DT-EGF-treated cells show no caspase activity, DT-EGF causes caspase activity in Atg5 siRNA-treated cells that is similar to the level achieved by treatment with Fas ligand plus cycloheximide.
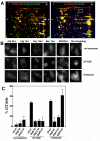
Figure 5. Autophagy regulates selective release of HMGB1 from DT-EGF-treated cells
Panel A, U87MG cells transfected with GFP-HMGB1 and control (non-specific) or Atg12 siRNAs were monitored by time-lapse microscopy after treatment with DT-EGF. DT-EGF causes release of HMGB1 in control cells, Atg12 knockdown causes the retention of HMGB1 in fragmented apoptotic nuclei. Panel B, western blot analysis of cell extract and media for HMGB1 protein after DT-EGF treatment for 48 hours. HMGB1 is not released into the media in DT-EGF-treated cells when autophagy regulators are knocked down. Control shows cells treated for 3.5 hours with 10mM H2O2.
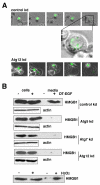
Figure 6. DT-EGF treatment causes HMGB1 release without causing membrane lysis and necrosis
Panel A, propidium iodide staining of U87MG cells after treatment with DT-EGF, 10mM H2O2, or DT-EGF after knockdown of Atg5. DT-EGF alone does not cause increased propidium iodide staining compared with living, untreated cells and displays less propidium iodide staining than in DT-EGF cells with Atg5 knockdown. Panel B, Quantitation of percentage of propidium iodide positive nuclei after treatment with DT-EGF, H2O2, or DT-EGF after knockdown of Atg5 (mean +/- SD from 3 replicates). Panel C, lactate dehydrogenase (LDH) release in U87 MG cells treated with DT-EGF or 10mM H2O2, no LDH is released in DT-EGF-treated cells (mean +/- SD from three replicates). Panel D, western analysis of HMGB1 in cells and media from HN12 epithelial cells treated with DT-EGF in the presence or absence of trehalose to stimulate autophagy; trehalose treatment promotes HMGB1 release into the media. Panel E, LDH release in the HN12 cells after treatment with DT-EGF with or without trehalose cells; trehalose treatment does not increase LDH release (mean +/- SD from three replicates).
Similar articles
- Regulation of HMGB1 release by autophagy.
Thorburn J, Frankel AE, Thorburn A. Thorburn J, et al. Autophagy. 2009 Feb;5(2):247-9. doi: 10.4161/auto.5.2.7552. Epub 2009 Feb 5. Autophagy. 2009. PMID: 19098461 Free PMC article. - Diphtheria toxin-epidermal growth factor fusion protein and Pseudomonas exotoxin-interleukin 13 fusion protein exert synergistic toxicity against human glioblastoma multiforme cells.
Liu TF, Willingham MC, Tatter SB, Cohen KA, Lowe AC, Thorburn A, Frankel AE. Liu TF, et al. Bioconjug Chem. 2003 Nov-Dec;14(6):1107-14. doi: 10.1021/bc034111+. Bioconjug Chem. 2003. PMID: 14624623 - A diphtheria toxin-epidermal growth factor fusion protein is cytotoxic to human glioblastoma multiforme cells.
Liu TF, Cohen KA, Ramage JG, Willingham MC, Thorburn AM, Frankel AE. Liu TF, et al. Cancer Res. 2003 Apr 15;63(8):1834-7. Cancer Res. 2003. PMID: 12702570 - Autophagy-mediated HMGB1 release promotes gastric cancer cell survival via RAGE activation of extracellular signal-regulated kinases 1/2.
Zhang QY, Wu LQ, Zhang T, Han YF, Lin X. Zhang QY, et al. Oncol Rep. 2015 Apr;33(4):1630-8. doi: 10.3892/or.2015.3782. Epub 2015 Feb 4. Oncol Rep. 2015. PMID: 25652880 Free PMC article. - Decoding cell death signals in liver inflammation.
Brenner C, Galluzzi L, Kepp O, Kroemer G. Brenner C, et al. J Hepatol. 2013 Sep;59(3):583-94. doi: 10.1016/j.jhep.2013.03.033. Epub 2013 Apr 6. J Hepatol. 2013. PMID: 23567086 Review.
Cited by
- The influence of sex on neuroimmune communication, pain, and physiology.
Alexander SN, Green AR, Debner EK, Ramos Freitas LE, Abdelhadi HMK, Szabo-Pardi TA, Burton MD. Alexander SN, et al. Biol Sex Differ. 2024 Oct 22;15(1):82. doi: 10.1186/s13293-024-00660-w. Biol Sex Differ. 2024. PMID: 39439003 Free PMC article. Review. - Emerging degrader technologies engaging lysosomal pathways.
Ding Y, Xing D, Fei Y, Lu B. Ding Y, et al. Chem Soc Rev. 2022 Oct 31;51(21):8832-8876. doi: 10.1039/d2cs00624c. Chem Soc Rev. 2022. PMID: 36218065 Free PMC article. Review. - Immunological Significance of HMGB1 Post-Translational Modification and Redox Biology.
Kwak MS, Kim HS, Lee B, Kim YH, Son M, Shin JS. Kwak MS, et al. Front Immunol. 2020 Jun 10;11:1189. doi: 10.3389/fimmu.2020.01189. eCollection 2020. Front Immunol. 2020. PMID: 32587593 Free PMC article. Review. - Autophagy and metastasis: another double-edged sword.
Kenific CM, Thorburn A, Debnath J. Kenific CM, et al. Curr Opin Cell Biol. 2010 Apr;22(2):241-5. doi: 10.1016/j.ceb.2009.10.008. Epub 2009 Nov 27. Curr Opin Cell Biol. 2010. PMID: 19945838 Free PMC article. Review. - Autophagy Modulation by Viral Infections Influences Tumor Development.
Leonardi L, Sibéril S, Alifano M, Cremer I, Joubert PE. Leonardi L, et al. Front Oncol. 2021 Oct 22;11:743780. doi: 10.3389/fonc.2021.743780. eCollection 2021. Front Oncol. 2021. PMID: 34745965 Free PMC article. Review.
References
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21(Nov 15)(22):2861–2873. - PubMed
- Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Nov)(Suppl 2):1528–1534. - PubMed
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120(Jan 28)(2):237–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA111421/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- R01 CA111421-01/CA/NCI NIH HHS/United States
- R01 CA111421/CA/NCI NIH HHS/United States
- R01 CA111421-04/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials