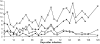Trypanosoma brucei rhodesiense transmitted by a single tsetse fly bite in vervet monkeys as a model of human African trypanosomiasis - PubMed (original) (raw)
Trypanosoma brucei rhodesiense transmitted by a single tsetse fly bite in vervet monkeys as a model of human African trypanosomiasis
John K Thuita et al. PLoS Negl Trop Dis. 2008.
Abstract
We have investigated the pathogenicity of tsetse (Glossina pallidipes)-transmitted cloned strains of Trypanosoma brucei rhodesiense in vervet monkeys. Tsetse flies were confirmed to have mature trypanosome infections by xenodiagnosis, after which nine monkeys were infected via the bite of a single infected fly. Chancres developed in five of the nine (55.6%) monkeys within 4 to 8 days post infection (dpi). All nine individuals were successfully infected, with a median pre-patent period of 4 (range = 4-10) days, indicating that trypanosomes migrated from the site of fly bite to the systemic circulation rapidly and independently of the development of the chancre. The time lag to detection of parasites in cerebrospinal fluid (CSF) was a median 16 (range = 8-40) days, marking the onset of central nervous system (CNS, late) stage disease. Subsequently, CSF white cell numbers increased above the pre-infection median count of 2 (range = 0-9) cells/microl, with a positive linear association between their numbers and that of CSF trypanosomes. Haematological changes showed that the monkeys experienced an early microcytic-hypochromic anaemia and severe progressive thrombocytopaenia. Despite a 3-fold increase in granulocyte numbers by 4 dpi, leucopaenia occurred early (8 dpi) in the monkey infection, determined mainly by reductions in lymphocyte numbers. Terminally, leucocytosis was observed in three of nine (33%) individuals. The duration of infection was a median of 68 (range = 22-120) days. Strain and individual differences were observed in the severity of the clinical and clinical pathology findings, with two strains (KETRI 3741 and 3801) producing a more acute disease than the other two (KETRI 3804 and 3928). The study shows that the fly-transmitted model accurately mimics the human disease and is therefore a suitable gateway to understanding human African trypanosomiasis (HAT; sleeping sickness).
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Figure 1. Parasitaemia curves generated by four T.b. rhodesiense cloned strains after experimental fly (Glossina pallidipes) infection of nine vervet monkeys (+-476, □-515, ♦-536, ◊-523, ▪-579, ▴-556, x-574, •-554, ▵-555).
After a variable pre-patent period, the parasitaemia tended to plateau but with more clearly defined waves of relapse and recrudescence in the individuals with longer disease duration.
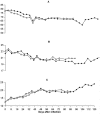
Figure 2. Changes in the mean corpuscular volume (MCV) mean corpuscular haemoglobin (MCH) and Red cell distribution width (RDW) in two vervet monkeys (•-554 and ▵-555) that were infected with T.b. rhodesiense KETRI 3928.
Panel A: MCV; Panel B: MCH; and Panel C: RDW. Note that while both MCV and MCH decreased below pre-infection levels and remained low throughout; RDW increased throughout the infection.
Figure 3. Changes in total and differential white blood cell numbers in vervet 554 that was infected with T.b. rhodesiense KETRI 3928.
During the early part of the infection, granulocytes and lymphocytes are approximately equal while later in the infection, lymphocytes are clearly the predominant cell type. (X- Total WBC, ▴-lymphocytes, ▵-Granulocytes, ▪-Monocytes).
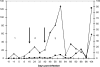
Figure 4. Changes in cerebrospinal fluid (CSF) trypanosomes (▪) and white cell (▴) during the course of infection of vervet 476 with T.b. rhodesiense KETRI 3741.
Phase 1: early stage infection; Phase 2: transition of intermediate stage infection and phase 3: late stage disease which at terminal point was marked by surge in trypanosome numbers and white cell counts.
Similar articles
- Mouse experiments demonstrate differential pathogenicity and virulence of Trypanosoma brucei rhodesiense strains.
Kipkorir LW, John TK, Owino OB, John O, Robert S, Daniel M, Owino AV. Kipkorir LW, et al. Exp Parasitol. 2021 Sep;228:108135. doi: 10.1016/j.exppara.2021.108135. Epub 2021 Jul 17. Exp Parasitol. 2021. PMID: 34284027 Free PMC article. - Influence of trypanocidal therapy on the haematology of vervet monkeys experimentally infected with Trypanosoma brucei rhodesiense.
Ngotho M, Kagira JM, Kariuki C, Maina N, Thuita JK, Mwangangi DM, Farah IO, Hau J. Ngotho M, et al. Acta Trop. 2011 Jul;119(1):14-8. doi: 10.1016/j.actatropica.2011.02.013. Epub 2011 Mar 21. Acta Trop. 2011. PMID: 21420376 - Spatial distribution and trypanosome infection of tsetse flies in the sleeping sickness focus of Zimbabwe in Hurungwe District.
Shereni W, Anderson NE, Nyakupinda L, Cecchi G. Shereni W, et al. Parasit Vectors. 2016 Nov 25;9(1):605. doi: 10.1186/s13071-016-1879-5. Parasit Vectors. 2016. PMID: 27884172 Free PMC article. - [Human African trypanosomiasis].
Dumas M, Bouteille B. Dumas M, et al. C R Seances Soc Biol Fil. 1996;190(4):395-408. C R Seances Soc Biol Fil. 1996. PMID: 8952890 Review. French. - Tsetse Flies (Glossina) as Vectors of Human African Trypanosomiasis: A Review.
Wamwiri FN, Changasi RE. Wamwiri FN, et al. Biomed Res Int. 2016;2016:6201350. doi: 10.1155/2016/6201350. Epub 2016 Feb 29. Biomed Res Int. 2016. PMID: 27034944 Free PMC article. Review.
Cited by
- Trypanosoma brucei metabolite indolepyruvate decreases HIF-1α and glycolysis in macrophages as a mechanism of innate immune evasion.
McGettrick AF, Corcoran SE, Barry PJ, McFarland J, Crès C, Curtis AM, Franklin E, Corr SC, Mok KH, Cummins EP, Taylor CT, O'Neill LA, Nolan DP. McGettrick AF, et al. Proc Natl Acad Sci U S A. 2016 Nov 29;113(48):E7778-E7787. doi: 10.1073/pnas.1608221113. Epub 2016 Nov 15. Proc Natl Acad Sci U S A. 2016. PMID: 27856732 Free PMC article. - New insights in staging and chemotherapy of African trypanosomiasis and possible contribution of medicinal plants.
Seke Etet PF, Mahomoodally MF. Seke Etet PF, et al. ScientificWorldJournal. 2012;2012:343652. doi: 10.1100/2012/343652. Epub 2012 Apr 19. ScientificWorldJournal. 2012. PMID: 22593674 Free PMC article. - Vaccination against trypanosomiasis: can it be done or is the trypanosome truly the ultimate immune destroyer and escape artist?
La Greca F, Magez S. La Greca F, et al. Hum Vaccin. 2011 Nov;7(11):1225-33. doi: 10.4161/hv.7.11.18203. Hum Vaccin. 2011. PMID: 22205439 Free PMC article. Review. - Efficacy of the novel diamidine compound 2,5-Bis(4-amidinophenyl)- furan-bis-O-Methlylamidoxime (Pafuramidine, DB289) against Trypanosoma brucei rhodesiense infection in vervet monkeys after oral administration.
Mdachi RE, Thuita JK, Kagira JM, Ngotho JM, Murilla GA, Ndung'u JM, Tidwell RR, Hall JE, Brun R. Mdachi RE, et al. Antimicrob Agents Chemother. 2009 Mar;53(3):953-7. doi: 10.1128/AAC.00831-08. Epub 2008 Dec 8. Antimicrob Agents Chemother. 2009. PMID: 19064893 Free PMC article. - Safety, pharmacokinetic, and efficacy studies of oral DB868 in a first stage vervet monkey model of human African trypanosomiasis.
Thuita JK, Wolf KK, Murilla GA, Liu Q, Mutuku JN, Chen Y, Bridges AS, Mdachi RE, Ismail MA, Ching S, Boykin DW, Hall JE, Tidwell RR, Paine MF, Brun R, Wang MZ. Thuita JK, et al. PLoS Negl Trop Dis. 2013 Jun 6;7(6):e2230. doi: 10.1371/journal.pntd.0002230. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23755309 Free PMC article.
References
- Jennings FW, Whitelaw DD, Urquhart GM. The relationship between duration of infection with Trypanosoma brucei in mice and the efficacy of chemotherapy. J Parasitol. 1977;75:143–153. - PubMed
- Baker JR, Taylor ER. Experimental infections of the Chimpanzee (Pan Troglodytes) with Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. Ann Trop Med Parasitol. 1971;65:471–485. - PubMed
- Schmidt H, Sayer P. T. b. rhodesiense infection in vervet monkeys. II. Provocation of the encephalitic late phase by treatment of infected monkeys. Tropenmed Parasitol. 1982;33:255–259. - PubMed
- Bouteille B, Millet P, Enanga B, Mezui MJ, Keita M, Jauberteau MO, Georges A, Dumas M. Human African trypanosomiasis: contributions of experimental models. Bull Soc Pathol Exot. 1998;91:127–132. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical