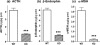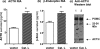Major role of cathepsin L for producing the peptide hormones ACTH, beta-endorphin, and alpha-MSH, illustrated by protease gene knockout and expression - PubMed (original) (raw)
Major role of cathepsin L for producing the peptide hormones ACTH, beta-endorphin, and alpha-MSH, illustrated by protease gene knockout and expression
Lydiane Funkelstein et al. J Biol Chem. 2008.
Abstract
The pituitary hormones adrenocorticotropic hormone (ACTH), beta-endorphin, and alpha-melanocyte stimulating hormone (alpha-MSH) are synthesized by proteolytic processing of their common proopiomelanocortin (POMC) precursor. Key findings from this study show that cathepsin L functions as a major proteolytic enzyme for the production of POMC-derived peptide hormones in secretory vesicles. Specifically, cathepsin L knock-out mice showed major decreases in ACTH, beta-endorphin, and alpha-MSH that were reduced to 23, 18, and 7% of wild-type controls (100%) in pituitary. These decreased peptide levels were accompanied by increased levels of POMC consistent with proteolysis of POMC by cathepsin L. Immunofluorescence microscopy showed colocalization of cathepsin L with beta-endorphin and alpha-MSH in the intermediate pituitary and with ACTH in the anterior pituitary. In contrast, cathepsin L was only partially colocalized with the lysosomal marker Lamp-1 in pituitary, consistent with its extralysosomal function in secretory vesicles. Expression of cathepsin L in pituitary AtT-20 cells resulted in increased ACTH and beta-endorphin in the regulated secretory pathway. Furthermore, treatment of AtT-20 cells with CLIK-148, a specific inhibitor of cathepsin L, resulted in reduced production of ACTH and accumulation of POMC. These findings demonstrate a prominent role for cathepsin L in the production of ACTH, beta-endorphin, and alpha-MSH peptide hormones in the regulated secretory pathway.
Figures
FIGURE 1.
Cathepsin L knock-out mice show substantial reduction of POMC-derived ACTH, β-endorphin, and α-MSH peptide hormones in pituitary. Pituitaries from cathepsin L knock-out mice (eight mice) and from wild-type control mice (10 mice) were dissected and prepared as acid extracts for radioimmune assay quantitation of the peptide hormones ACTH, β-endorphin, and α-MSH with results shown in a, b, and c, respectively. The mean ± S.E. are shown. Comparisons of cathepsin L KO with wild-type (WT) mice showed highly significant differences (indicated by ***) between these groups for ACTH, β-endorphin, and α-MSH, which showed p < 0.001 (by Student's t test).
FIGURE 2.
Accumulation of POMC in pituitary of cathepsin L KO mice. a, anti-ACTH Western blots reveal accumulation of POMC and 22-kDa product in cathepsin L KO mice. Analyses by anti-ACTH Western blots of pituitary extracts from cathepsin L KO and wild-type mice were performed to investigate relative levels of POMC. Representative blots are shown for two samples of pituitary extracts from two KO mice and from two wild-type (WT) mice (25 μg of protein/lane). Cathepsin L KO mice showed increases in intact POMC and the POMC-derived 22-kDa ACTH intermediate.b, POMC-derived 22-kDa intermediate that contains ACTH. An illustration of the POMC prohormone shows the predicted domains for ACTH, β-endorphin, α-MSH, and the 22-kDa ACTH intermediate.
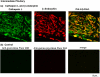
FIGURE 3.
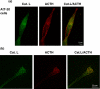
In vivo colocalization of cathepsin L with β-endorphin present in secretory vesicles of intermediate pituitary. a, colocalization of cathepsin L with β-endorphin. The colocalization of cathepsin L with β-endorphin in intermediate pituitary (mouse) was demonstrated by immunofluorescence confocal microscopy. Cathepsin L immunoreactivity (green fluorescence) showed excellent overlapping colocalization with β-endorphin (red fluorescence), shown by the_yellow fluorescence_ of merged cathepsin L/β-endorphin fluorescent immunostaining. The majority of β-endorphin, contained in secretory vesicles, was colocalized with cathepsin L. b, controls show lack of immunostaining with only secondary antibody. As control, tissue sections were incubated only secondary antibody, without primary antisera to peptide hormones. The secondary anti-goat-Alexa Fluor 488 alone (without anti-cathepsin L) resulted in a lack of immunofluorescence staining. The secondary anti-goat-Alexa Fluor 594 (without anti-β-endorphin) also resulted in lack of immunofluorescence staining. The merged image of these two secondary antisera showed lack of immunofluorescence staining. These controls illustrate specific immunofluorescence staining by the primary antisera to the peptide hormones (a).
FIGURE 4.
Colocalization of cathepsin L with α-MSH present in secretory vesicles of intermediate pituitary. The overlapping colocalization of cathepsin L with α-MSH peptide hormone in intermediate pituitary was illustrated by immunofluorescence confocal microscopy. The majority of cathepsin L (green fluorescence) and α-MSH (red fluorescence) was colocalized, shown by the merged areas of yellow fluorescence. Controls with only secondary antisera showed lack of immunofluorescence staining (like the results of Fig. 3_b_). Thus, primary antisera detected cathepsin L colocalization with α-MSH.
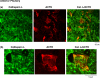
FIGURE 5.
In vivo colocalization of cathepsin L with ACTH present in secretory vesicles of anterior pituitary. ACTH-containing cells of anterior pituitary were indicated by ACTH immunofluorescence staining (red fluorescence). The areas of ACTH showed colocalization with cathepsin L (green fluorescence), shown by the merged areas of yellow fluorescence. It is known that only a few percent of anterior pituitary cells are represented by the corticotroph cells that contain ACTH. The_upper_ and lower sections (a) and (b) represent images of different magnification (see distance bars), with_b_ showing a larger magnification. Controls with only secondary antisera showed lack of immunofluorescence staining. Thus, results show colocalization of cathepsin L with ACTH.
FIGURE 6.
Lack of complete localization of cathepsin L with the lysosomal marker Lamp-1 in pituitary. Comparison of the cellular distribution of cathepsin L in intermediate lobe pituitary cells was compared with that of the lysosomal marker Lamp-1. Punctate-like immunofluorescence staining of cathepsin L (green fluorescence) was only partially colocalized with Lamp-1 (red fluorescence). This subcellular distribution of cathepsin L is consistent with the localization of cathepsin L in secretory vesicles as well as in lysosomes. Controls with only secondary antisera showed lack of immunofluorescence staining. Results show cathepsin L localization to extralysosomal areas of the cell, consistent with its observed localization in secretory vesicles.
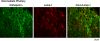
FIGURE 7.
Cathepsin L colocalization with ACTH in AtT-20 mouse pituitary cells. a, colocalization of cathepsin L with ACTH in the AtT-20 pituitary cell line. Cathepsin L in the AtT-20 mouse pituitary cell line was colocalized with ACTH, demonstrated by dual imunofluorescence confocal microscopy (a and b). Cathepsin L (green fluorescence) showed a punctate pattern of subcellular immunostaining that showed overlapping localization with areas of ACTH immunostaining (red fluorescence), shown by the merged images, with yellow fluorescence indicating colocalization. Controls with only secondary antisera showed the absence of immunofluorescence staining. b, cathepsin L localization with ACTH in neuritic extensions of AtT-20 cells. Examination of the neuritic extensions of AtT-20 cells shows the presence of cathepsin L colocalized with ACTH. Controls with only secondary antisera showed lack of immunofluorescence staining.
FIGURE 8.
Expression of cathepsin L increases production of ACTH andβ-endorphin in the regulated secretory pathway of AtT-20 cells. a and b, ACTH and β-endorphin production by cathepsin L. Expression of cathepsin L (bovine) in AtT-20 cells by transfection resulted in increased amounts of ACTH (a) and β-endorphin (b) produced in regulated secretory vesicles whose contents were released in the presence of BaCl2. Cells were transfected at 70% confluence with cathepsin L/pcDNA3.1 or pcDNA3.1 vector alone, and 2 days later, cells were incubated in BaCl2 for 2 h, and the medium was collected for radioimmune assay measurements of ACTH and β-endorphin. BaCl2 is a stimulator of regulated secretion (22). Expression of cathepsin L resulted in increased production of ACTH and β-endorphin in regulated secretory vesicles, whose secretion was observed in the presence of BaCl2. Results are shown as the mean ± S.E.(n = 6 for each group, and the experiment was repeated twice) with significant increases of ACTH and β-endorphin produced after expression of cathepsin with p < 0.05 (by Student's t test). c, cathepsin L expression and analysis by anti-ACTH Western blots. Western blots with anti-ACTH of AtT-20 cells transfected with the cathepsin L cDNA (lane 2) or with control vector without cDNA (lane 1) showed that cathepsin L-transfected cells displayed increased amounts of ACTH (lane 2)(2μg of protein/lane).
FIGURE 9.
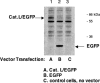
Expression of cathepsin L/EGFP fusion protein. Expression of cathepsin L by the CMV-driven promoter of the expression plasmid was monitored by demonstrating expression of the cathepsin L/EGFP fusion protein in the pEGFP-NI plasmid expression vector, which distinguishes expressed cathepsin L from endogenous cathepsin L. Two days after transfection, expression of cathepsin L/EGFP was monitored by Western blots with anti-EGFP. Western blots showed expression of cathepsin L/EGFP of an apparent molecular mass (∼60 kDa) (lane 1) that is consistent with the combined molecular masses of cathepsin L and EGFP (60 kDa) (indicated by an arrow). The ∼60 kDa band was absent in cells transfected with pEGFP-NI plasmid alone (no cathepsin L) (lane 2); these cells demonstrated expression of EGFP (indicated by an arrow). Cells that were not subjected to transfection did not show cathepsin L/EGFP or EGFP bands (lane 3). It is noted that a band at ∼55 kDa probably represents a nonspecific band detected by anti-EGFP, since it is present in untransfected and transfected cells in all three lanes. Comparison of lane 1 with lanes 2 and 3 demonstrates expression of cathepsin L/EGFP (lane 1).
FIGURE 10.
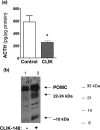
CLIK-148, inhibitor of cathepsin L, reduces ACTH production in AtT-20 cells. a, reduction of ACTH after treatment with CLIK-148. After treatment of AtT-20 cells with and without CLIK-148 (for 5 days), ACTH content in cells (in acetic acid extracts) was measured by a radioimmune assay.*, statistical significance between inhibitor and control groups (p < 0.05, based on Student's t test). b, CLIK-148 treatment results in accumulation of POMC and a 22-kDa intermediate. Anti-ACTH Western blots of cells (5 μg of protein/lane) of CLIK-148-treated cells showed accumulation of POMC (lane 2) compared with untreated control cells (lane 1). In addition, 22- and 10-kDa intermediates also accumulated in CLIK-148-treated cells compared with untreated controls.
Similar articles
- Human pituitary contains dual cathepsin L and prohormone convertase processing pathway components involved in converting POMC into the peptide hormones ACTH, alpha-MSH, and beta-endorphin.
Hook V, Funkelstein L, Toneff T, Mosier C, Hwang SR. Hook V, et al. Endocrine. 2009 Jun;35(3):429-37. doi: 10.1007/s12020-009-9163-5. Epub 2009 Apr 3. Endocrine. 2009. PMID: 19343278 Free PMC article. - Obliteration of alpha-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice.
Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld MC, Hook VY. Miller R, et al. J Neurochem. 2003 Aug;86(3):556-63. doi: 10.1046/j.1471-4159.2003.01856.x. J Neurochem. 2003. PMID: 12859669 - 60 YEARS OF POMC: Purification and biological characterisation of melanotrophins and corticotrophins.
Lowry P. Lowry P. J Mol Endocrinol. 2016 May;56(4):T1-T12. doi: 10.1530/JME-15-0260. Epub 2015 Dec 7. J Mol Endocrinol. 2016. PMID: 26643914 Review. - [Corticotrope].
Tanaka K. Tanaka K. Nihon Rinsho. 1993 Oct;51(10):2606-10. Nihon Rinsho. 1993. PMID: 8254928 Review. Japanese.
Cited by
- Spinal astrocytes produce and secrete dynorphin neuropeptides.
Wahlert A, Funkelstein L, Fitzsimmons B, Yaksh T, Hook V. Wahlert A, et al. Neuropeptides. 2013 Apr;47(2):109-15. doi: 10.1016/j.npep.2012.10.006. Epub 2013 Jan 3. Neuropeptides. 2013. PMID: 23290538 Free PMC article. - Cathepsin Protease Controls Copper and Cisplatin Accumulation via Cleavage of the Ctr1 Metal-binding Ectodomain.
Öhrvik H, Logeman B, Turk B, Reinheckel T, Thiele DJ. Öhrvik H, et al. J Biol Chem. 2016 Jul 1;291(27):13905-13916. doi: 10.1074/jbc.M116.731281. Epub 2016 May 3. J Biol Chem. 2016. PMID: 27143361 Free PMC article. - Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing.
Gupta N, Bark SJ, Lu WD, Taupenot L, O'Connor DT, Pevzner P, Hook V. Gupta N, et al. J Proteome Res. 2010 Oct 1;9(10):5065-75. doi: 10.1021/pr100358b. J Proteome Res. 2010. PMID: 20704348 Free PMC article. - Design of Gallinamide A Analogs as Potent Inhibitors of the Cysteine Proteases Human Cathepsin L and Trypanosoma cruzi Cruzain.
Boudreau PD, Miller BW, McCall LI, Almaliti J, Reher R, Hirata K, Le T, Siqueira-Neto JL, Hook V, Gerwick WH. Boudreau PD, et al. J Med Chem. 2019 Oct 24;62(20):9026-9044. doi: 10.1021/acs.jmedchem.9b00294. Epub 2019 Oct 4. J Med Chem. 2019. PMID: 31539239 Free PMC article. - Neuropeptidomic components generated by proteomic functions in secretory vesicles for cell-cell communication.
Hook V, Bark S, Gupta N, Lortie M, Lu WD, Bandeira N, Funkelstein L, Wegrzyn J, O'Connor DT, Pevzner P. Hook V, et al. AAPS J. 2010 Dec;12(4):635-45. doi: 10.1208/s12248-010-9223-z. Epub 2010 Aug 24. AAPS J. 2010. PMID: 20734175 Free PMC article. Review.
References
- Nakanishi, S., lnoue, A., Kita, T., Nakamura, M., Chang, A. C. Y., Cohen, S. N., and Numa, S. (1979) Nature 278 423-424 - PubMed
- Frohman, L. A. (1981) in Endocrinology and Metabolism (Felig, P., Baxter, J. D., and Frohman, L. A., eds) pp. 293-312, McGraw-Hill, Inc., New York
- Norris, D. O. (1997) Vertebrate Endocrinology, pp. 106-166, Academic Press, Inc., San Diego
- Lechan, R. M. and Fekete, C. (2006) Peptides 27 310-325 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 NS047101/NS/NINDS NIH HHS/United States
- R01 DA 04271/DA/NIDA NIH HHS/United States
- R01 MH 077305/MH/NIMH NIH HHS/United States
- R01 NS 24553/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous