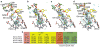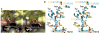Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase - PubMed (original) (raw)
doi: 10.1038/nchembio.115. Epub 2008 Oct 12.
Andrew S Arvai, Robin J Rosenfeld, Matt D Kroeger, Brian R Crane, Gunilla Andersson, Glen Andrews, Peter J Hamley, Philip R Mallinder, David J Nicholls, Stephen A St-Gallay, Alan C Tinker, Nigel P Gensmantel, Antonio Mete, David R Cheshire, Stephen Connolly, Dennis J Stuehr, Anders Aberg, Alan V Wallace, John A Tainer, Elizabeth D Getzoff
Affiliations
- PMID: 18849972
- PMCID: PMC2868503
- DOI: 10.1038/nchembio.115
Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase
Elsa D Garcin et al. Nat Chem Biol. 2008 Nov.
Abstract
Nitric oxide synthase (NOS) enzymes synthesize nitric oxide, a signal for vasodilatation and neurotransmission at low concentrations and a defensive cytotoxin at higher concentrations. The high active site conservation among all three NOS isozymes hinders the design of selective NOS inhibitors to treat inflammation, arthritis, stroke, septic shock and cancer. Our crystal structures and mutagenesis results identified an isozyme-specific induced-fit binding mode linking a cascade of conformational changes to a new specificity pocket. Plasticity of an isozyme-specific triad of distant second- and third-shell residues modulates conformational changes of invariant first-shell residues to determine inhibitor selectivity. To design potent and selective NOS inhibitors, we developed the anchored plasticity approach: anchor an inhibitor core in a conserved binding pocket, then extend rigid bulky substituents toward remote specificity pockets, which become accessible upon conformational changes of flexible residues. This approach exemplifies general principles for the design of selective enzyme inhibitors that overcome strong active site conservation.
Figures
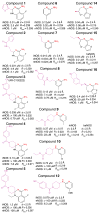
Figure 1. NOS inhibitors structures, inhibition and crystallographic data
For all inhibitors, including quinazolines (left column: compounds 1–5), aminopyridines (middle column: compounds 6–12) and bicyclic thienooxazepines (right column: compounds 14–16), the chemical structure is shown in black (core with red cis-amidine nitrogens) and magenta (tail), together with IC50 values in the three human NOS isozymes. The resolution (d in Å), crystallographic R and Rfree values are indicated for each structure of murine iNOSox (unlabeled), human iNOSox (hiNOS), bovine eNOSox (beNOS) and human eNOSox (heNOS) complexes.
Figure 2
Quinazoline and aminopyridine binding in iNOSox and eNOSox. (a) Potent but non-selective aminopyridine compound 6 (ref. 28) bound to murine iNOSox. (b) Highly-selective quinazoline compound 3 (ref. 26) bound to murine iNOSox. (c) Selective aminopyridine compound 12 (ref. 28) bound to murine iNOSox. (d) Aminopyridine 9 (ref. 28) bound to human iNOSox. For all structures, critical hydrogen bonds (dots) and iNOS residues are shown: active-site residues (peach), first-shell residues (yellow, residues interacting directly with the inhibitor), second-shell residues (orange, residues interacting with first-shell residues) and third-shell residues (green, residues interacting with second-shell residues). The Fo-Fc electron density map contoured at 3 σ (blue mesh) is shown around each inhibitor (pink). (e) Key iNOS residues involved in inhibitor binding are colored according to a–d.
Figure 3
Selective aminopyridine compound 9 binding to eNOS versus iNOS. (a) Solvent-accessible surfaces for the iNOS (left) and eNOS (right) active sites colored according to Fig. 2. The core of compound 9 binds closer and more parallel to the heme in eNOS. In iNOS, side-chain rotations of Gln, Arg, and Arg388 open the Gln specificity pocket for binding of the bulky inhibitor tail. (b) Stereoview of the superimposition of bovine eNOS:compound 9 (yellow) and human iNOS:compound 9 (blue) x-ray structures, highlighting the cascade of conformational changes of first-shell and second-shell residues upon inhibitor binding to iNOS.
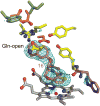
Figure 4
Isozyme-specific induced-fit upon inhibitor binding. Schematic of the cascade of conformational changes associated with inhibitor binding in the three human NOS isozymes. The van der Waals surfaces for the isozyme-specific triads are shown in orange (second shell) and green (third shell). In human iNOS (hiNOS), inhibitor binding first induces the Gln-closed to Gln-open conformation and Arg rotation, which in turn leads to rotation of second-shell Asn towards third-shell Phe286 and Val305. In human eNOS (heNOS), bulkier third-shell residues (Ile269 and Leu288) prevent the Asn rotation (overlap of van der Waals surfaces). In human nNOS (hnNOS), partial rotation of Asn towards third-shell residues Phe506 and bulky Leu525 may be possible.
Figure 5
Bicyclic thioenooxazepine inhibitor binding in iNOSox. Moderately selective compound 16 binds to murine iNOSox similarly to bulky quinazoline and aminopyridine inhibitors and induces the Gln-open conformation. Residues are colored according to Fig. 2. The Fo-Fc electron density map contoured at 3 σ (blue mesh) is shown around the inhibitor (pink).
Similar articles
- Inhibitor Bound Crystal Structures of Bacterial Nitric Oxide Synthase.
Holden JK, Dejam D, Lewis MC, Huang H, Kang S, Jing Q, Xue F, Silverman RB, Poulos TL. Holden JK, et al. Biochemistry. 2015 Jul 7;54(26):4075-82. doi: 10.1021/acs.biochem.5b00431. Epub 2015 Jun 23. Biochemistry. 2015. PMID: 26062720 Free PMC article. - Structures of nitric oxide synthase isoforms complexed with the inhibitor AR-R17477 suggest a rational basis for specificity and inhibitor design.
Fedorov R, Vasan R, Ghosh DK, Schlichting I. Fedorov R, et al. Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5892-7. doi: 10.1073/pnas.0306588101. Epub 2004 Apr 7. Proc Natl Acad Sci U S A. 2004. PMID: 15071192 Free PMC article. - 1,2-Dihydro-4-quinazolinamines: potent, highly selective inhibitors of inducible nitric oxide synthase which show antiinflammatory activity in vivo.
Tinker AC, Beaton HG, Boughton-Smith N, Cook TR, Cooper SL, Fraser-Rae L, Hallam K, Hamley P, McInally T, Nicholls DJ, Pimm AD, Wallace AV. Tinker AC, et al. J Med Chem. 2003 Mar 13;46(6):913-6. doi: 10.1021/jm0255926. J Med Chem. 2003. PMID: 12620067 - Computational studies of competitive inhibitors of nitric oxide synthase (NOS) enzymes: towards the development of powerful and isoform-selective inhibitors.
Tafi A, Angeli L, Venturini G, Travagli M, Corelli F, Botta M. Tafi A, et al. Curr Med Chem. 2006;13(16):1929-46. doi: 10.2174/092986706777585031. Curr Med Chem. 2006. PMID: 16842203 Review. - Design of isoform-selective inhibitors of nitric oxide synthase.
Babu BR, Griffith OW. Babu BR, et al. Curr Opin Chem Biol. 1998 Aug;2(4):491-500. doi: 10.1016/s1367-5931(98)80125-7. Curr Opin Chem Biol. 1998. PMID: 9736922 Review.
Cited by
- The structural biochemistry of the superoxide dismutases.
Perry JJ, Shin DS, Getzoff ED, Tainer JA. Perry JJ, et al. Biochim Biophys Acta. 2010 Feb;1804(2):245-62. doi: 10.1016/j.bbapap.2009.11.004. Epub 2009 Nov 13. Biochim Biophys Acta. 2010. PMID: 19914407 Free PMC article. Review. - Exploration of the active site of neuronal nitric oxide synthase by the design and synthesis of pyrrolidinomethyl 2-aminopyridine derivatives.
Ji H, Delker SL, Li H, Martásek P, Roman LJ, Poulos TL, Silverman RB. Ji H, et al. J Med Chem. 2010 Nov 11;53(21):7804-24. doi: 10.1021/jm100947x. J Med Chem. 2010. PMID: 20958055 Free PMC article. - Homocysteine disrupts outgrowth of microvascular endothelium by an iNOS-dependent mechanism.
Mayo JN, Chen CH, Liao FF, Bearden SE. Mayo JN, et al. Microcirculation. 2014 Aug;21(6):541-50. doi: 10.1111/micc.12133. Microcirculation. 2014. PMID: 24655004 Free PMC article. - An efficient chemical screening method for structure-based inhibitors to nucleic acid enzymes targeting the DNA repair-replication interface and SARS CoV-2.
Moiani D, Link TM, Brosey CA, Katsonis P, Lichtarge O, Kim Y, Joachimiak A, Ma Z, Kim IK, Ahmed Z, Jones DE, Tsutakawa SE, Tainer JA. Moiani D, et al. Methods Enzymol. 2021;661:407-431. doi: 10.1016/bs.mie.2021.09.003. Epub 2021 Sep 27. Methods Enzymol. 2021. PMID: 34776222 Free PMC article. - Essential Oils from the Leaves, Stem, and Roots of Blumea lanceolaria (Roxb.) Druce in Vietnam: Determination of Chemical Composition, and In Vitro, In Vivo, and In Silico Studies on Anti-Inflammatory Activity.
Do TTH, Nguyen TU, Nguyen TTH, Ho TY, Pham TLH, Le TS, Nguyen THV, Nguyen PH, Nguyen QH, Nguyen VS. Do TTH, et al. Molecules. 2022 Nov 14;27(22):7839. doi: 10.3390/molecules27227839. Molecules. 2022. PMID: 36431950 Free PMC article.
References
- Geller DA, Billiar TR. Molecular Biology of nitric oxide synthases. Cancer and Metastasis Rev. 1998;17:7–23. - PubMed
- Nathan C. The moving frontier in nitric oxide-dependent signaling. Science STKE. 2004;2004:pe52. - PubMed
- Bian K, Murad F. Nitric oxide (NO)--biogeneration, regulation, and relevance to human diseases. Front Biosci. 2003;8:d264–78. - PubMed
- Thippeswamy T, McKay JS, Quinn JP, Morris R. Nitric oxide, a biological double-faced Janus--is this good or bad? Histol Histopathol. 2006;21:445–458. - PubMed
- Duncan AJ, Heales SJ. Nitric oxide and neurological disorders. Mol Aspects Med. 2005;26:67–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA053914-17A1/CA/NCI NIH HHS/United States
- R01 GM051491-13A2/GM/NIGMS NIH HHS/United States
- R01 CA053914/CA/NCI NIH HHS/United States
- R01 HL058883-10/HL/NHLBI NIH HHS/United States
- R01 GM051491/GM/NIGMS NIH HHS/United States
- R01 HL058883/HL/NHLBI NIH HHS/United States
- R01 CA053914-19/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Chemical Information
Molecular Biology Databases