PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice - PubMed (original) (raw)
. 2008 Dec;11(12):1392-401.
doi: 10.1038/nn.2220. Epub 2008 Oct 8.
Affiliations
- PMID: 18849983
- PMCID: PMC3842596
- DOI: 10.1038/nn.2220
PDGFRA/NG2 glia generate myelinating oligodendrocytes and piriform projection neurons in adult mice
Leanne E Rivers et al. Nat Neurosci. 2008 Dec.
Abstract
Platelet-derived growth factor alpha receptor (PDGFRA)/NG2-expressing glia are distributed throughout the adult CNS. They are descended from oligodendrocyte precursors (OLPs) in the perinatal CNS, but it is not clear whether they continue to generate myelinating oligodendrocytes or other differentiated cells during normal adult life. We followed the fates of adult OLPs in Pdgfra-creER(T2)/Rosa26-YFP double-transgenic mice and found that they generated many myelinating oligodendrocytes during adulthood; >20% of all oligodendrocytes in the adult mouse corpus callosum were generated after 7 weeks of age, raising questions about the function of the late-myelinating axons. OLPs also produced some myelinating cells in the cortex, but the majority of adult-born cortical cells did not appear to myelinate. We found no evidence for astrocyte production in gray or white matter. However, small numbers of projection neurons were generated in the forebrain, especially in the piriform cortex, which is the main target of the olfactory bulb.
Figures
Figure 1
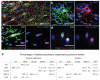
Antigenic properties of adult OLPs/NG2 cells. (a–e) Cryosections of young adult (P45) brain were double-immunolabeled for different combinations of oligodendrocyte lineage markers PDGFRA (Ra), NG2, OLIG2, SOX10 and CNP. Representative images are shown in a–d, and cell counts (means ± s.d.) in e. Column headers refer to the labeled populations and row headers refer to the coexpressed second marker. Certain antibody combinations were not possible because of incompatibilities between the required fixation conditions or the species origin of the antibodies (see Methods). Other combinations were not possible because the available antibodies were incompatible. Excluding vascular cells, essentially all of the PDGFRA+ OLPs were colabeled for NG2 and vice-versa, both in corpus callosum (CC) and cortex (Ctx). NG2+ OLPs all colabeled for SOX10, but not all SOX10+ cells were NG2+, as SOX10 continued to be expressed in differentiated oligodendrocytes, whereas NG2 and PDGFRA were downregulated rapidly during differentiation. Scale bars represent 50 μm in a and 10 μm in b–d.
Figure 2
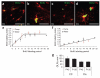
Cumulative BrdU labeling of adult OLPs/NG2 cells in vivo. BrdU was administered to young adult (P60) mice for up to 17 consecutive days via osmotic mini-pumps or by including BrdU in the drinking water for up to 21 d (see Methods). (a–d) Double-immunolabeling brain sections for BrdU and PDGFRA revealed double-positive (replicating) OLPs in both corpus callosum and cerebral cortex (Ctx). Both routes of BrdU delivery gave comparable data. (e) In corpus callosum, the BrdU labeling index increased to ~0.55 in 7–10 d, but did not increase further. (f) In the cortex, the labeling index increased linearly for 21 d until ~40% of OLPs were BrdU+. At each time point, the labeling index was determined from cell counts (means ± s.d.) on at least three sections from each of three animals. The 2-h time point is from a single intra-peritoneal injection of BrdU (see Methods). (g) The numbers of PDGFRA+ OLPs per unit area were counted in corpus callosum and cortex at P45 and P135 (14-μm sections). There was no significant change (P > 0.7) in OLP cell number or density between these ages. Scale bars represent 45 μm in a, 15 μm in b and d and 40 μm in c.
Figure 3
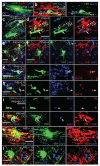
PDGFRA+ adult OLPs generate differentiated oligodendrocyte lineage cells. (a–c) Sections of P45 tamoxifen-induced Pdgfra-creERT2/Rosa26-YFP mice were immunolabeled for YFP and PDGFRA or NG2 at P45 + 5 (arrows in a indicate YFP+ PDGFRA+ OLPs (yellow), arrowhead indicates a PDGFRA+ YFP− OLP (red)). (d) The fraction of PDGFRA+ cells that coexpressed YFP increased between P45 + 5 and P45 + 8, and then remained constant until at least P45 + 90, indicating that Cre recombination continues for 1–4 d after the final dose of tamoxifen. With our protocol, ~45% of OLPs labeled for YFP after P45 + 8. The fraction of YFP+ cells that coexpressed PDGFRA and NG2 was high at P45 + 3, but declined with time post-tamoxifen (e), as increasing numbers of YFP+, PDGFRA− cells appeared (f–h) (arrows in g indicate YFP+ PDGFRA+ OLPs, yellow; arrowhead indicates a YFP+ PDGFRA− cell, green). This suggests that PDGFRA+ OLPs differentiate and downregulate PDGFRA. (h) YFP+ PDGFRA+ and YFP+ PDGFRA− cells in the cortex. The rate of addition of PDGFRA− (differentiated) cells was higher at P45 than at P180 and was higher in corpus callosum than in cortex at either age (f). (i,j) The subpopulation of OLPs that were labeled for YFP was not biased toward either dividing or nondividing OLPs, as the BrdU labeling index of YFP+ PDGFRA+ cells matched that of the PDGFRA+ population overall. Between P45 + 5 and P45 + 210, all YFP-labeled cells were SOX10+ oligodendrocyte lineage cells, both in the corpus callosum (k,l) and motor cortex (data not shown). Scale bars represent 35 μm in a, g, k and l, and 10 μm in b, c, h and j.
Figure 4
Evidence for myelin protein synthesis in adult-born oligodendrocytes. Tamoxifen was administered to P45 Pdgfra-creERT2/Rosa26-YFP mice and forebrain sections were prepared and immunolabeled for YFP and PDGFRA, SOX10, CNP or MBP to identify adult-born (that is, YFP+) oligodendrocyte lineage cells. (a–d) In the corpus callosum at P45 + 28, YFP+ SOX10+ cells possessed many thin parallel cytoplasmic extensions resembling the cytoplasmic tongue processes of myelinating oligodendrocytes (a), which were aligned with neurofilament+ axons (b). In the corpus callosum at P45 + 8, some YFP+ PDGFRA− cells (arrowhead in c) expressed CNP in their cell bodies and proximal processes (arrows in d). (e,f) YFP+ PDGFRA+ OLPs did not colabel for CNP. (g,h) Cell bodies that were triple labeled for YFP, SOX10 and CNP were found at later times (for example, P45 + 210) in the corpus callosum (g) and cortex (h). (i,j) We exposed tamoxifen-induced P60 Pdgfra-creERT2/Rosa26-YFP mice to BrdU via the drinking water for 21 d and analyzed them on P140. Many BrdU+ YFP+ cells in corpus callosum and cortex were PDGFRA− CNP+ (data not shown), confirming that differentiated oligodendrocytes had been generated from dividing PDGFRA+ precursors. (k,l) Some YFP+ cell processes also colabeled for MBP at P45 + 10 (arrows). Scale bars represent 10 μm in a–c, e, k and l), and 20 μm in d, f, and g–j.
Figure 5
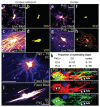
Adult OLPs generate myelinating oligodendrocytes. To reveal full cell morphology, we used YFP in live tissue slices to target cells for Alexa Fluor dye injection via glass patch pipettes. (a–d) Cells with the distinctive morphology of myelinating oligodendrocytes, with up to 50 rod-like myelin internodes, were identified in the corpus callosum (c,d), as well as presumptive OLPs with slender, multiply branching processes (a,b). (e,f) In the cortical gray matter, the large majority of live dye-filled cells resembled OLPs. (g,h) A few filled cells in cortical layers 5/6 did possess some myelin-like structures (arrowheads in g); a digital reconstruction gives a clearer impression of whole cell morphology (h). (i,j) To quantify the number of cells with myelinating morphology in the corpus callosum, we filled YFP+ cells in fixed tissue slices with DiI. This allowed larger numbers of cells to be examined, but the DiI did not perfuse the cells as completely as did Alexa dye in live cells. Nevertheless, we could distinguish cells with progenitor morphology (i) versus myelinating morphology (j). (k) The proportion of myelinating cells increased with time post-tamoxifen in the corpus callosum; at P45 + 75 roughly 70% of YFP+ cells had myelinating morphology. (l) We immunolabeled fix-filled cells with antibody to MBP and showed that dye-filled processes contained MBP, indicating that they were indeed myelin internodes. Scale bars represent 10 μm.
Figure 6
Adult OLPs do not generate astrocytes. Sections of P45 + 90 Pdgfra-creERT2/Rosa26-YFP mice were immunolabeled for YFP and GFAP. (a–d) No double-labeled cells were observed anywhere in the corpus callosum or cortex. Sections were also immunolabeled for YFP and S100β, a marker of protoplasmic astrocytes and some oligodendrocyte lineage cells. (e) In cortex, some YFP+ S100β+ cells were present, but these invariably colabeled for PDGFRA (yellow arrow), identifying them as OLPs. (f) In the corpus callosum, all of the YFP+ S100β+ cells were SOX10+ (yellow arrow in f). The white arrow in f indicates a YFP−,SOX10+ S100β+ oligodendrocyte lineage cell and the white arrowhead indicates a YFP− SOX10−,S100β+ presumptive astrocyte. Scale bars represent 10 μm in b and e, and 25 μm in a, c, d and f.
Figure 7
OLPs generate cortical projection neurons in vivo. (a–l) Pdgfra-creERT2/Rosa26-YFP mice were tamoxifen-induced at P45 and sections were immunolabeled for YFP and oligodendrocyte and/or neuronal lineage markers. In the ventral forebrain, occasional YFP+ SOX10− cells appeared (arrowheads in a and c; SOX10+ cells indicated by arrows). These cells coexpressed the neuronal marker NeuN (b–e,j,l), but were PDGFRA− and OLIG2− (arrowheads in d and e). The hypothalamus is depicted in a, but all other fields are from layers 2/3 of the aPC (red territory in f, inset). We followed the accumulation of YFP+ NeuN+ cells in the aPC over time (numbers of neurons per 30-μm section, mean ± s.d., n = 3 mice, f). Most YFP+ NeuN+ cells appeared after a delay of 28–90 d and continued to accumulate after that. In parallel, we examined Fgfr3-icreERT2/Rosa26-YFP mice, in which all SVZ stem cells and many protoplasmic astrocytes were labeled for YFP (Supplementary Fig. 3). YFP+ NeuN+ neurons did not accumulate in the aPC of these mice (f), supporting the conclusion that the adult-born neurons are formed from cells outside the SVZ. Some YFP+ cell processes colabeled for MAP2 (g) and TAU1 (h,i). We were unable to label YFP+ NeuN+ neurons with BrdU (arrows in j,l). A BrdU+ YFP+ OLP acts as a control (arrowheads in j and k). Scale bars represent 10 μm in g, 20 μm in b and d, 30 μm in a, c, e and j) and 40 μm in h.
Figure 8
Morphologies of YFP-labeled neurons in the piriform cortex. (a) NeuN+ neurons in Pdgfra-creERT2/Rosa26-YFP mice were frequently located in layer 2 of the piriform cortex (primary olfactory cortex), with processes projecting into the lateral olfactory tract under the pial surface. In all micrographs the pial surface is top-right. Two types of principal neurons have been described in layer 2; semilunar neurons and superficial pyramidal neurons, which receive inputs from the olfactory bulbs via the lateral olfactory tract. (b–d) Semilunar neurons are often located more superficially than superficial pyramidal neurons (layer 2a and 2b, respectively). From morphology and their positions in layer 2, we speculate that the YFP+ NeuN+ neurons that we observe correspond to semilunar (b) and superficial pyramidal (c,d) neurons. Scale bars represent 30 μm.
Comment in
- Glial progenitor cells in the adult brain reveal their alternate fate.
Kang SH, Bergles DE. Kang SH, et al. Nat Neurosci. 2008 Dec;11(12):1365-7. doi: 10.1038/nn1208-1365. Nat Neurosci. 2008. PMID: 19023339 No abstract available.
Similar articles
- Early postnatal proteolipid promoter-expressing progenitors produce multilineage cells in vivo.
Guo F, Ma J, McCauley E, Bannerman P, Pleasure D. Guo F, et al. J Neurosci. 2009 Jun 3;29(22):7256-70. doi: 10.1523/JNEUROSCI.5653-08.2009. J Neurosci. 2009. PMID: 19494148 Free PMC article. - Pyramidal neurons are generated from oligodendroglial progenitor cells in adult piriform cortex.
Guo F, Maeda Y, Ma J, Xu J, Horiuchi M, Miers L, Vaccarino F, Pleasure D. Guo F, et al. J Neurosci. 2010 Sep 8;30(36):12036-49. doi: 10.1523/JNEUROSCI.1360-10.2010. J Neurosci. 2010. PMID: 20826667 Free PMC article. - Cell cycle dynamics of NG2 cells in the postnatal and ageing brain.
Psachoulia K, Jamen F, Young KM, Richardson WD. Psachoulia K, et al. Neuron Glia Biol. 2009 Nov;5(3-4):57-67. doi: 10.1017/S1740925X09990354. Neuron Glia Biol. 2009. PMID: 20346197 Free PMC article. - NG2-glia, More Than Progenitor Cells.
Eugenín-von Bernhardi J, Dimou L. Eugenín-von Bernhardi J, et al. Adv Exp Med Biol. 2016;949:27-45. doi: 10.1007/978-3-319-40764-7_2. Adv Exp Med Biol. 2016. PMID: 27714683 Review. - SomethiNG 2 talk about-Transcriptional regulation in embryonic and adult oligodendrocyte precursors.
Küspert M, Wegner M. Küspert M, et al. Brain Res. 2016 May 1;1638(Pt B):167-182. doi: 10.1016/j.brainres.2015.07.024. Epub 2015 Jul 29. Brain Res. 2016. PMID: 26232072 Review.
Cited by
- Phenotype overlap in glial cell populations: astroglia, oligodendroglia and NG-2(+) cells.
Alghamdi B, Fern R. Alghamdi B, et al. Front Neuroanat. 2015 May 12;9:49. doi: 10.3389/fnana.2015.00049. eCollection 2015. Front Neuroanat. 2015. PMID: 26106302 Free PMC article. - A new role for the P2Y-like GPR17 receptor in the modulation of multipotency of oligodendrocyte precursor cells in vitro.
Boccazzi M, Lecca D, Marangon D, Guagnini F, Abbracchio MP, Ceruti S. Boccazzi M, et al. Purinergic Signal. 2016 Dec;12(4):661-672. doi: 10.1007/s11302-016-9530-7. Epub 2016 Aug 20. Purinergic Signal. 2016. PMID: 27544384 Free PMC article. - Ionic transporter activity in astrocytes, microglia, and oligodendrocytes during brain ischemia.
Annunziato L, Boscia F, Pignataro G. Annunziato L, et al. J Cereb Blood Flow Metab. 2013 Jul;33(7):969-82. doi: 10.1038/jcbfm.2013.44. Epub 2013 Apr 3. J Cereb Blood Flow Metab. 2013. PMID: 23549380 Free PMC article. Review. - EED-mediated histone methylation is critical for CNS myelination and remyelination by inhibiting WNT, BMP, and senescence pathways.
Wang J, Yang L, Dong C, Wang J, Xu L, Qiu Y, Weng Q, Zhao C, Xin M, Lu QR. Wang J, et al. Sci Adv. 2020 Aug 12;6(33):eaaz6477. doi: 10.1126/sciadv.aaz6477. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32851157 Free PMC article. - Neuroblasts contribute to oligodendrocytes generation upon demyelination in the adult mouse brain.
El Waly B, Bertet C, Paris M, Falque M, Milpied P, Magalon K, Cayre M, Durbec P. El Waly B, et al. iScience. 2022 Sep 13;25(10):105102. doi: 10.1016/j.isci.2022.105102. eCollection 2022 Oct 21. iScience. 2022. PMID: 36185360 Free PMC article.
References
- Hall A, Giese NA, Richardson WD. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development. 1996;122:4085–4094. - PubMed
- Butt AM, et al. PDGF-alpha receptor and myelin basic protein mRNAs are not coexpressed by oligodendrocytes in vivo: a double in situ hybridization study in the anterior medullary velum of the neonatal rat. Mol. Cell. Neurosci. 1997;8:311–322. - PubMed
- Nishiyama A, Chang A, Trapp BD. NG2+ glial cells: a novel glial cell population in the adult brain. J. Neuropathol. Exp. Neurol. 1999;58:1113–1124. - PubMed
- Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. - PubMed
- Wolswijk G, Noble M. Identification of an adult-specific glial progenitor cell. Development. 1989;105:387–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G0800575/MRC_/Medical Research Council/United Kingdom
- G9708005/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- G0501173/MRC_/Medical Research Council/United Kingdom
- 080513/WT_/Wellcome Trust/United Kingdom
- BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous