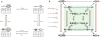A quantitative model of transcription factor-activated gene expression - PubMed (original) (raw)
. 2008 Nov;15(11):1192-8.
doi: 10.1038/nsmb.1500. Epub 2008 Oct 12.
Affiliations
- PMID: 18849996
- PMCID: PMC2696132
- DOI: 10.1038/nsmb.1500
A quantitative model of transcription factor-activated gene expression
Harold D Kim et al. Nat Struct Mol Biol. 2008 Nov.
Abstract
A challenge facing biology is to develop quantitative, predictive models of gene regulation. Eukaryotic promoters contain transcription factor binding sites of differing affinity and accessibility, but we understand little about how these variables combine to generate a fine-tuned, quantitative transcriptional response. Here we used the PHO5 promoter in budding yeast to quantify the relationship between transcription factor input and gene expression output, termed the gene-regulation function (GRF). A model that captures variable interactions between transcription factors, nucleosomes and the promoter faithfully reproduced the observed quantitative changes in the GRF that occur upon altering the affinity of transcription factor binding sites, and implicates nucleosome-modulated accessibility of transcription factor binding sites in increasing the diversity of gene expression profiles. This work establishes a quantitative framework that can be applied to predict GRFs of other eukaryotic genes.
Figures
Figure 1
Measuring the GRF from the tetracycline-regulated gene expression system. A variant of a tetracycline-controlled reverse transactivator is constitutively expressed under the control of the MYO2 promoter (PMYO2). When bound to doxycycline, it can activate the TETO7 promoter (PTETO7, containing seven repeats of the tetO operator sequence) and drive the expression of PHO4-YFP, which in turn activates the expression of CFP from a PHO5 promoter variant (PPHO5*). Pho4SA1-4PA6 used in this study is a constitutively nuclear form of Pho4 that has serine-to-alanine substitutions at phosphorylation sites 1, 2, 3 and 4, and a proline-to-alanine substitution at site 6 (ref. 16). Nhp6a, a chromatin protein in the nucleus, was also tagged with RFP to mark the nucleus and to serve as an internal standard for intensity normalization. The chromatin architectures of the PHO5 promoter variants are shown, with nucleosomes (Nuc) −3, −2 and −1 drawn as ellipses. Nucleosome −1 is the closest to the PHO5 open reading frame and occludes the TATA box, which is depicted as a white square with the letter T. Low-affinity (●) and high-affinity (▲) Pho4 binding sites differ by 1 bp out of a 6-bp core sequence (CACGTT versus CACGTG). Variant 4 shown inside the box is the wild-type PHO5 promoter, with a low-affinity site (UASp1) in the exposed region between nucleosome −3 and −2, and a high-affinity site (UASp2) under nucleosome −2. Variants 1, 2, 4, 8, 9 and 12 are labeled as LX, LL, LH, HX, HL and HH, respectively, where H, L and X are the high-affinity sites, the low-affinity site and no site, respectively.
Figure 2
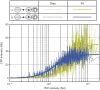
Raw data of fluorescence intensities from single cells and the fits to GRFs. YFP and CFP intensities were obtained from single cells, normalized to RFP intensity and plotted as dashed lines in arbitrary normalized units (NU). As an example, the GRFs of variant 4 and variant 9 are shown in gold and blue, respectively. Variant 4 (LH) is the wild-type strain, and variant 9 (HL) is obtained by swapping UASp1 and UASp2. The Hill curves fit to the data are shown as solid lines in corresponding colors.
Figure 3
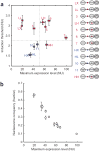
The diversity of GRFs and the relationship between the maximum expression level and the nucleosome occupancy over the TATA box region. (a) The GRF of each strain is represented by its maximum expression level on the x axis and its induction threshold on the y axis (NU, normalized unit). The labeling of the promoter variants is the same as in Figure 1. Error bars are one s.d. from a minimum of five different measurements. The variants are grouped by their similarity to one of the four representative variants—LX, LH, HX and HH, colored purple, red, blue and brown, respectively. The distinct features in the promoter architecture of each group are also highlighted in the corresponding group color. The segregation of variants by the horizontal and vertical dashed lines suggests that the induction threshold is determined by the affinity of the exposed binding site (LX and LH versus HX and HH), whereas the maximum expression level depends strongly on the affinity of the binding site under nucleosome −2 (LX and HX versus LH and HH). (b) The normalized histone H3 occupancy was measured by ChIP and plotted against the maximum expression level for each strain. The error bars are one s.d. of six different points, coming from three independent measurements normalized in two different ways (Methods). The inverse relationship supports the idea that the nucleosome occupancy over the TATA box region limits gene expression.
Figure 4
Quantitative models of Pho4-dependent chromatin remodeling. (a) This minimal model describes a promoter composed of one exposed Pho4 binding site and one adjacent nucleosome containing the TATA box. The presence or absence of Pho4 and the nucleosome (Nuc) are marked by O or X in the table, the combination of which defines the four states in this model. The orange pentagon represents Pho4, and all other symbols are as defined in Figure 1. The two states on the right are considered to be transcriptionally active as the general transcription machinery can access the TATA box region. The transitional frequencies of association, dissociation, remodeling and reassembly are described by kassoc∗, _k_dissoc, _k_remod and _k_reass, respectively. (b) This model expands on the minimal model in a to describe the variants LX, LL, LH, HX, HL and HH. The six states on the right comprise the transcriptionally active fraction. The rate constants in this model are for the association of Pho4 to DNA (kassoc∗), dissociation from the exposed (kdissocexp) or nucleosomal region (kdissocnuc), remodeling of nucleosome −2 or −1 (_k_remod) and the reassembly of nucleosome −2 or −1 (_k_reass). The arrow scheme for these rate constants is shown on the left. The entire model with three layers of squares is the model with nucleosome −2, and the model with the inner two layers highlighted in green corresponds to the model without nucleosome −2.
Figure 5
Comparison between the data and the model prediction. (a) We globally fitted six threshold values and six maximum expression levels from variants LX, LL, LH, HX, HL and HH using two different models—those with and without nucleosome −2. The experimental data (blue squares with error bars) are compared to the results from the model with nucleosome −2 (purple circles) and the model without nucleosome −2 (red circles). The promoter variants used are listed on the left axis. (b) Gene expression landscapes from the models with and without nucleosome −2. The maximum expression level and the induction threshold are plotted as a function of k¯dissocnuc (x axis) and k¯dissocexp (y axis) as heat maps. The other parameters were set to the values obtained from the global fit (Supplementary Discussion). The color scale is shown next to each heat map (AU, arbitrary unit). k¯dissocnuc and k¯dissocexp values of the six variants determined by the global fitting are plotted on each heat map: LX (●), LL (▼), LH (◄), HX (■), HL (▲) and HH (►). For these variants, k¯dissocexp can be either _k̄_H or _k̄_L, and k¯dissocnuc can be _k̄_H, _k̄_Lor infinity (∞). _k̄_H and _k̄_L are 0.17 and 1.27, respectively, with nucleosome −2, and 0.34 and 2.76, respectively, without nucleosome −2. For convenience, k¯dissocnuc = infinity is plotted as the maximum k¯dissocnuc value shown in the heat map. Nucleosome −2, by occluding one of the transcription factor binding sites, breaks the anti-correlated change of the maximum expression level and the induction threshold, thus increasing the variety of gene expression profiles.
Similar articles
- Chromatin-dependent transcription factor accessibility rather than nucleosome remodeling predominates during global transcriptional restructuring in Saccharomyces cerevisiae.
Zawadzki KA, Morozov AV, Broach JR. Zawadzki KA, et al. Mol Biol Cell. 2009 Aug;20(15):3503-13. doi: 10.1091/mbc.e09-02-0111. Epub 2009 Jun 3. Mol Biol Cell. 2009. PMID: 19494041 Free PMC article. - DNA Topoisomerases maintain promoters in a state competent for transcriptional activation in Saccharomyces cerevisiae.
Pedersen JM, Fredsoe J, Roedgaard M, Andreasen L, Mundbjerg K, Kruhøffer M, Brinch M, Schierup MH, Bjergbaek L, Andersen AH. Pedersen JM, et al. PLoS Genet. 2012;8(12):e1003128. doi: 10.1371/journal.pgen.1003128. Epub 2012 Dec 20. PLoS Genet. 2012. PMID: 23284296 Free PMC article. - Activator control of nucleosome occupancy in activation and repression of transcription.
Bryant GO, Prabhu V, Floer M, Wang X, Spagna D, Schreiber D, Ptashne M. Bryant GO, et al. PLoS Biol. 2008 Dec 23;6(12):2928-39. doi: 10.1371/journal.pbio.0060317. PLoS Biol. 2008. PMID: 19108605 Free PMC article. - Transcription factors vs nucleosomes: regulation of the PHO5 promoter in yeast.
Svaren J, Hörz W. Svaren J, et al. Trends Biochem Sci. 1997 Mar;22(3):93-7. doi: 10.1016/s0968-0004(97)01001-3. Trends Biochem Sci. 1997. PMID: 9066259 Review. - The yeast PHO5 promoter: from single locus to systems biology of a paradigm for gene regulation through chromatin.
Korber P, Barbaric S. Korber P, et al. Nucleic Acids Res. 2014;42(17):10888-902. doi: 10.1093/nar/gku784. Epub 2014 Sep 4. Nucleic Acids Res. 2014. PMID: 25190457 Free PMC article. Review.
Cited by
- The WD40-repeat proteins NFC101 and NFC102 regulate different aspects of maize development through chromatin modification.
Mascheretti I, Battaglia R, Mainieri D, Altana A, Lauria M, Rossi V. Mascheretti I, et al. Plant Cell. 2013 Feb;25(2):404-20. doi: 10.1105/tpc.112.107219. Epub 2013 Feb 19. Plant Cell. 2013. PMID: 23424244 Free PMC article. - Bacterial nucleoid-associated protein uncouples transcription levels from transcription timing.
Zwir I, Yeo WS, Shin D, Latifi T, Huang H, Groisman EA. Zwir I, et al. mBio. 2014 Oct 7;5(5):e01485-14. doi: 10.1128/mBio.01485-14. mBio. 2014. PMID: 25293763 Free PMC article. - De novo identification and biophysical characterization of transcription-factor binding sites with microfluidic affinity analysis.
Fordyce PM, Gerber D, Tran D, Zheng J, Li H, DeRisi JL, Quake SR. Fordyce PM, et al. Nat Biotechnol. 2010 Sep;28(9):970-5. doi: 10.1038/nbt.1675. Epub 2010 Aug 29. Nat Biotechnol. 2010. PMID: 20802496 Free PMC article. - Canonical and single-cell Hi-C reveal distinct chromatin interaction sub-networks of mammalian transcription factors.
Ma X, Ezer D, Adryan B, Stevens TJ. Ma X, et al. Genome Biol. 2018 Oct 25;19(1):174. doi: 10.1186/s13059-018-1558-2. Genome Biol. 2018. PMID: 30359306 Free PMC article. - Comprehensive, high-resolution binding energy landscapes reveal context dependencies of transcription factor binding.
Le DD, Shimko TC, Aditham AK, Keys AM, Longwell SA, Orenstein Y, Fordyce PM. Le DD, et al. Proc Natl Acad Sci U S A. 2018 Apr 17;115(16):E3702-E3711. doi: 10.1073/pnas.1715888115. Epub 2018 Mar 27. Proc Natl Acad Sci U S A. 2018. PMID: 29588420 Free PMC article.
References
- Cyert MS. Regulation of nuclear localization during signaling. J Biol Chem. 2001;276:20805–20808. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases