Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies - PubMed (original) (raw)
Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies
Josep Dalmau et al. Lancet Neurol. 2008 Dec.
Abstract
Background: A severe form of encephalitis associated with antibodies against NR1-NR2 heteromers of the NMDA receptor was recently identified. We aimed to analyse the clinical and immunological features of patients with the disorder and examine the effects of antibodies against NMDA receptors in neuronal cultures.
Methods: We describe the clinical characteristics of 100 patients with encephalitis and NR1-NR2 antibodies. HEK293 cells ectopically expressing single or assembled NR1-NR2 subunits were used to determine the epitope targeted by the antibodies. Antibody titres were measured with ELISA. The effect of antibodies on neuronal cultures was determined by quantitative analysis of NMDA-receptor clusters.
Findings: Median age of patients was 23 years (range 5-76 years); 91 were women. All patients presented with psychiatric symptoms or memory problems; 76 had seizures, 88 unresponsiveness (decreased consciousness), 86 dyskinesias, 69 autonomic instability, and 66 hypoventilation. 58 (59%) of 98 patients for whom results of oncological assessments were available had tumours, most commonly ovarian teratoma. Patients who received early tumour treatment (usually with immunotherapy) had better outcome (p=0.004) and fewer neurological relapses (p=0.009) than the rest of the patients. 75 patients recovered or had mild deficits and 25 had severe deficits or died. Improvement was associated with a decrease of serum antibody titres. The main epitope targeted by the antibodies is in the extracellular N-terminal domain of the NR1 subunit. Patients' antibodies decreased the numbers of cell-surface NMDA receptors and NMDA-receptor clusters in postsynaptic dendrites, an effect that could be reversed by antibody removal.
Interpretation: A well-defined set of clinical characteristics are associated with anti-NMDA-receptor encephalitis. The pathogenesis of the disorder seems to be mediated by antibodies.
Figures
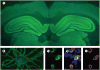
Figure 1. Immunohistochemical criteria for the presence of NR1–NR2B antibodies
Sera and CSF from all patients’ with anti-NMDA-receptor encephalitis showed identical antibody reactivity in three different assays. Coronal section of rat brain incubated with a representative CSF (A) shows intense reactivity predominantly involving the hippocampus. Cultures of non-permeabilised live rat hippocampal neurons (B) incubated with the same CSF show extensive cell-surface immunolabelling. HEK293 cells transfected with NR1 and NR2B (forming NR1–NR2B heteromers of the NMDA receptor) show intense reactivity with patients’ CSF (C); this reactivity co-localises (D) with the reactivity of a monoclonal rabbit antibody against NR1 (E). Immunofluorescence method, nuclei of cells shown with 4′,6-diamidino-2-phenylindole (DAPI). A ×25; B ×800 oil lens; C–E ×400.
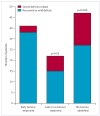
Figure 2. Response to treatment
Patients whose tumour was diagnosed and treated within 4 months of neurological symptom development had better outcomes (full recovery or mild deficits) than those whose tumour was treated after 4 months of neurological symptom development or not treated (p=0.03), those without tumour (p=0.006), and these two groups combined (p=0.004). See webtable 1 for more details.
Figure 3. Analysis of NR1 antibody titres
In 53 patients with anti-NMDA-receptor encephalitis, antibody titres were higher in CSF than in serum (A). In 83 patients with anti-NMDA-receptor encephalitis (54 with tumour, 29 without tumour) and 50 controls (B), those with tumours had higher titres than those without (Wilcoxon rank, p<0.0001) and controls (p<0.0001). Six patients (one with tumour, five without tumour) had very low ELISA readings that overlapped with the signal given by negative controls. These six patients had low antibody titres; in contrast, the 50 controls were negative. Solid lines indicate the mean of the titres in each group. The dotted line indicates three SD above the mean value given by background signal of negative controls. Follow-up of serum antibody titres (C) in 14 representative patients who had neurological improvement (black lines) and four who did not (red lines); the second time-point is the sample obtained at the last follow-up (median 5.6 months, range 2–83 months). The dotted line indicates three SD of the mean value given by background signal of 50 negative control sera. Similar results were obtained by ELISA with NR1–NR2 heteromers (data not shown). Values in A, B, and C are given in relative fluorescence units (rfu) from the ELISA reader, and plotted in a logarithmic scale.
Figure 4. Immunolabelling of neuronal NR1 clusters
Left: Hippocampal neurons (14 days in vitro) immunostained with antibody against NR1 (black and white and green) or a patient’s CSF (black and white and red). Right: 91% of NR1 clusters are colabelled with patient’s CSF (yellow puncta in overlay), less than 9% of NR1-positive puncta remain unlabelled (green in overlay). Kruskal-Wallis non-parametric ANOVA followed by Dunn’s pairwise comparison, p<0.01.
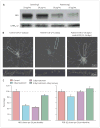
Figure 5. Effect of antibodies on the number of NMDA-receptor clusters in live neurons
Representative immunoblot (A) of neuronal surface NR1 detection after incubation with control or patients’ IgG (μg/mL). Protein concentrations were normalised to concentrations of GABAA receptor subunit a2. Hippocampal neurons (B) cultured with control CSF or patients’ CSF from day 7 to day 14 in vitro (7 day treatment), or with patients’ CSF from day 7 to day 10 in vitro followed by control CSF from day 11 to day 14 in vitro (3 day treatment and 4 day recovery), then immunostained for NR1 (18–36 cells from each of three experiments). Boxed areas are shown below at higher magnification. Fewer NR1-labelled clusters (C) were found in cultures treated with patient CSF for 3 or 7 days compared with those treated with control CSF or cultures treated with patient CSF followed by 4 days recovery (18–36 cells from each of three experiments; Kruskal-Wallis non-parametric ANOVA, Dunn’s pairwise comparison, p<0.01). Incubation with patients’ CSF for 3 days or 7 days did not affect the number of PSD-95 clusters (D; 18–36 cells from each of three experiments).
Comment in
- Anti-NMDA-receptor encephalitis: a cause of psychiatric, seizure, and movement disorders in young adults.
Vincent A, Bien CG. Vincent A, et al. Lancet Neurol. 2008 Dec;7(12):1074-5. doi: 10.1016/S1474-4422(08)70225-4. Epub 2008 Oct 11. Lancet Neurol. 2008. PMID: 18851929 No abstract available.
Similar articles
- Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma.
Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Dalmau J, et al. Ann Neurol. 2007 Jan;61(1):25-36. doi: 10.1002/ana.21050. Ann Neurol. 2007. PMID: 17262855 Free PMC article. Clinical Trial. - N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes.
Irani SR, Bera K, Waters P, Zuliani L, Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, Lang B, Bien CG, Vincent A. Irani SR, et al. Brain. 2010 Jun;133(Pt 6):1655-67. doi: 10.1093/brain/awq113. Brain. 2010. PMID: 20511282 Free PMC article. - Anti-NMDA-receptor encephalitis: a cause of psychiatric, seizure, and movement disorders in young adults.
Vincent A, Bien CG. Vincent A, et al. Lancet Neurol. 2008 Dec;7(12):1074-5. doi: 10.1016/S1474-4422(08)70225-4. Epub 2008 Oct 11. Lancet Neurol. 2008. PMID: 18851929 No abstract available. - [Anti-nMDA receptor encephalitis--clinical manifestations and pathophysiology].
Iizuka T, Sakai F. Iizuka T, et al. Brain Nerve. 2008 Sep;60(9):1047-60. Brain Nerve. 2008. PMID: 18807939 Review. Japanese. - [Anti-NMDA receptor antibody-mediated encephalitis/encephalopathy].
Iizuka T, Hara A. Iizuka T, et al. Rinsho Byori. 2009 Mar;57(3):252-61. Rinsho Byori. 2009. PMID: 19363996 Review. Japanese.
Cited by
- Prolonged Ketamine Effects in Sp4 Hypomorphic Mice: Mimicking Phenotypes of Schizophrenia.
Ji B, Wang X, Pinto-Duarte A, Kim M, Caldwell S, Young JW, Behrens MM, Sejnowski TJ, Geyer MA, Zhou X. Ji B, et al. PLoS One. 2013 Jun 18;8(6):e66327. doi: 10.1371/journal.pone.0066327. Print 2013. PLoS One. 2013. PMID: 23823008 Free PMC article. - Stroke patients develop antibodies that react with components of N-methyl-D-aspartate receptor subunit 1 in proportion to lesion size.
Kalev-Zylinska ML, Symes W, Little KC, Sun P, Wen D, Qiao L, Young D, During MJ, Barber PA. Kalev-Zylinska ML, et al. Stroke. 2013 Aug;44(8):2212-9. doi: 10.1161/STROKEAHA.113.001235. Epub 2013 May 30. Stroke. 2013. PMID: 23723305 Free PMC article. - Anti-N-methyl-d-aspartate encephalitis with ovarian cystadenofibroma.
Sanmaneechai O, Song JL, Nevadunsky N, Moshé SL, Overby PJ. Sanmaneechai O, et al. Pediatr Neurol. 2013 Mar;48(3):232-5. doi: 10.1016/j.pediatrneurol.2012.10.013. Pediatr Neurol. 2013. PMID: 23419475 Free PMC article. - Analysis of the Effect of Incentive Nursing Intervention in Children with Severe Viral Encephalitis and Myocarditis during Rehabilitation Based on Diffusion Weighted MRI.
Ren Q, Guo L, Liu X, Xiao P, Tang S, Sharma A, Walia TS, Shah MA. Ren Q, et al. J Healthc Eng. 2021 May 13;2021:9993264. doi: 10.1155/2021/9993264. eCollection 2021. J Healthc Eng. 2021. PMID: 34094044 Free PMC article. - Prospective Quantification of CSF Biomarkers in Antibody-Mediated Encephalitis.
Day GS, Yarbrough MY, Körtvelyessy P, Prüss H, Bucelli RC, Fritzler MJ, Mason W, Tang-Wai DF, Steriade C, Hébert J, Henson RL, Herries EM, Ladenson JH, Lopez-Chiriboga AS, Graff-Radford NR, Morris JC, Fagan A. Day GS, et al. Neurology. 2021 May 18;96(20):e2546-e2557. doi: 10.1212/WNL.0000000000011937. Epub 2021 Apr 1. Neurology. 2021. PMID: 33795390 Free PMC article.
References
- Lynch DR, Anegawa NJ, Verdoorn T, Pritchett DB. N-methyl-D-aspartate receptors: different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol Pharmacol. 1994;45:540–45. - PubMed
- Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. - PubMed
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56 CA089054/CA/NCI NIH HHS/United States
- R01 CA089054-06A2/CA/NCI NIH HHS/United States
- R56 MH057683/MH/NIMH NIH HHS/United States
- R01 CA107192-04/CA/NCI NIH HHS/United States
- R21 MH057683/MH/NIMH NIH HHS/United States
- 2R56CA089054/CA/NCI NIH HHS/United States
- R01 MH057683/MH/NIMH NIH HHS/United States
- R01 CA107192/CA/NCI NIH HHS/United States
- R01CA107192/CA/NCI NIH HHS/United States
- R01 CA089054/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical