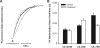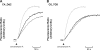Chloroquine resistance-conferring mutations in pfcrt give rise to a chloroquine-associated H+ leak from the malaria parasite's digestive vacuole - PubMed (original) (raw)
Chloroquine resistance-conferring mutations in pfcrt give rise to a chloroquine-associated H+ leak from the malaria parasite's digestive vacuole
Adele M Lehane et al. Antimicrob Agents Chemother. 2008 Dec.
Abstract
Chloroquine resistance in the malaria parasite Plasmodium falciparum is conferred by mutations in the P. falciparum chloroquine resistance transporter (PfCRT). PfCRT localizes to the membrane of the parasite's internal digestive vacuole, an acidic organelle in which chloroquine accumulates to high concentrations and exerts its toxic effect. Mutations in PfCRT are thought to reduce chloroquine accumulation in this organelle. How they do so is the subject of ongoing debate. Recently we have shown that in the presence of chloroquine there is an increased leak of H+ from the digestive vacuole in chloroquine-resistant but not chloroquine-sensitive parasites. Here, using transfectant parasite strains of a single genetic background and differing only in their pfcrt allele, we show that chloroquine resistance-conferring PfCRT mutations are responsible for this chloroquine-associated H+ leak. This is consistent with the hypothesis that the chloroquine resistance-conferring forms of PfCRT mediate the efflux of chloroquine, in association with H+, from the malaria parasite's digestive vacuole.
Figures
FIG. 1.
Alkalinization of the DV upon addition of the V-type H+-ATPase inhibitor concanamycin A. (A) Representative fluorometer traces for mature trophozoite-stage C2_GC03 (CQS) (dark gray), C4_Dd2 (CQR) (black), and C6_7G8 (CQR) (light gray) parasites. The parasites were isolated by saponin permeabilization of the erythrocyte membrane and suspended in minimal saline solution at pH 7.1 and 37°C. The addition of concanamycin A (100 nM) is indicated by the black arrowhead. The data are representative of those obtained in at least seven independent experiments for each strain, and the fluorescence ratio was normalized (to account for variations in signal intensity between experiments) by dividing by the maximum change in fluorescence ratio. (B) Averaged data for the rate of DV alkalinization following the addition of 100 nM concanamycin A (expressed as the inverse of the half-time for alkalinization) in the CQS strain C2_GC03 and in the CQR strains C4_Dd2 and C6_7G8 in the presence (white bars) and absence (black bars; solvent control) of 50 μM verapamil. The data (shown with the standard error of the mean) are from paired experiments (five for C2_GC03, six for C4_Dd2, and eight for C6_7G8).
FIG. 2.
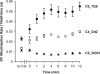
Effect of the length of time of CQ exposure on the rate of concanamycin A-induced DV alkalinization. CQ (2.5 μM) was added to suspensions of isolated mature trophozoite-stage C2_GC03 (CQS) (closed triangles), C4_Dd2 (CQR) (open circles), and C6_7G8 (CQR) (closed circles) parasites at the indicated number of minutes prior to the addition of 100 nM concanamycin A. The data are averaged from the results of four to five independent experiments for C2_GC03, two to five independent experiments for C4_Dd2, and four to seven independent experiments for C6_7G8 and are shown with the standard error of the mean. Where not shown, error bars fall within the symbols.
FIG. 3.
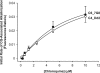
CQ concentration dependence of the CQ-associated increase in the initial rate of concanamycin A-induced DV alkalinization in isolated mature trophozoite-stage CQR C4_Dd2 (open circles) and C6_7G8 (closed circles) parasites. Initial rates of DV alkalinization were determined by fitting an exponential curve to the data {[F = _F_0 + a(1 − e_−_bt)], where F is the fluorescence ratio, _F_0 is the starting fluorescence ratio, t is time, and a and b are fitted constants}. Multiplication of a and b yielded the initial rate of alkalinization. To account for differences in signal intensity between experiments, the fluorescence ratio was normalized prior to determining the initial rate by dividing by the maximum change in fluorescence ratio. Within each experiment, the initial rate of alkalinization for the control trace without CQ was subtracted from the initial rates in the presence of CQ. The data are averaged from the results of five independent experiments for each strain; the lines were drawn using an exponential curve fitted to the data {[y = _y_0 + a(1 − e_−_bx)], where y is the initial rate of alkalinization; x is the CQ concentration; and _y_0, a, and b are fitted constants}. Error bars show standard errors of the means. For clarity, only positive error bars are shown for C6_7G8 and only negative error bars are shown for C4_Dd2.
FIG. 4.
Representative fluorometer traces showing DV alkalinization in isolated mature trophozoite-stage CQR C4_Dd2 (A) and C6_7G8 (B) parasites in the presence of 2.5 μM CQ (light gray), in the presence of 2.5 μM CQ and 50 μM verapamil (dark gray), and in the presence of 50 μM verapamil alone (black). The data are representative of the results of two independent experiments for C4_Dd2 and three independent experiments for C6_7G8.
Similar articles
- Efflux of a range of antimalarial drugs and 'chloroquine resistance reversers' from the digestive vacuole in malaria parasites with mutant PfCRT.
Lehane AM, Kirk K. Lehane AM, et al. Mol Microbiol. 2010 Aug;77(4):1039-51. doi: 10.1111/j.1365-2958.2010.07272.x. Epub 2010 Jun 28. Mol Microbiol. 2010. PMID: 20598081 - Chloroquine transport via the malaria parasite's chloroquine resistance transporter.
Martin RE, Marchetti RV, Cowan AI, Howitt SM, Bröer S, Kirk K. Martin RE, et al. Science. 2009 Sep 25;325(5948):1680-2. doi: 10.1126/science.1175667. Science. 2009. PMID: 19779197 - A 2-amino quinoline, 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid, interacts with PfMDR1 and inhibits its drug transport in Plasmodium falciparum.
Edaye S, Reiling SJ, Leimanis ML, Wunderlich J, Rohrbach P, Georges E. Edaye S, et al. Mol Biochem Parasitol. 2014 Jun;195(1):34-42. doi: 10.1016/j.molbiopara.2014.05.006. Epub 2014 Jun 8. Mol Biochem Parasitol. 2014. PMID: 24914817 - Is PfCRT a channel or a carrier? Two competing models explaining chloroquine resistance in Plasmodium falciparum.
Sanchez CP, Stein WD, Lanzer M. Sanchez CP, et al. Trends Parasitol. 2007 Jul;23(7):332-9. doi: 10.1016/j.pt.2007.04.013. Epub 2007 May 10. Trends Parasitol. 2007. PMID: 17493873 Review. - Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance.
Bray PG, Martin RE, Tilley L, Ward SA, Kirk K, Fidock DA. Bray PG, et al. Mol Microbiol. 2005 Apr;56(2):323-33. doi: 10.1111/j.1365-2958.2005.04556.x. Mol Microbiol. 2005. PMID: 15813727 Review.
Cited by
- Novel Baicalein-Derived Inhibitors of Plasmodium falciparum.
Gudla CS, Selvam V, Selvaraj SS, Tripathi R, Joshi P, Shaham SH, Singh M, Shandil RK, Habib S, Narayanan S. Gudla CS, et al. Pathogens. 2023 Oct 13;12(10):1242. doi: 10.3390/pathogens12101242. Pathogens. 2023. PMID: 37887758 Free PMC article. - pH-dependence of the Plasmodium falciparum chloroquine resistance transporter is linked to the transport cycle.
Berger F, Gomez GM, Sanchez CP, Posch B, Planelles G, Sohraby F, Nunes-Alves A, Lanzer M. Berger F, et al. Nat Commun. 2023 Jul 15;14(1):4234. doi: 10.1038/s41467-023-39969-2. Nat Commun. 2023. PMID: 37454114 Free PMC article. - Piperaquine-resistant PfCRT mutations differentially impact drug transport, hemoglobin catabolism and parasite physiology in Plasmodium falciparum asexual blood stages.
Okombo J, Mok S, Qahash T, Yeo T, Bath J, Orchard LM, Owens E, Koo I, Albert I, Llinás M, Fidock DA. Okombo J, et al. PLoS Pathog. 2022 Oct 28;18(10):e1010926. doi: 10.1371/journal.ppat.1010926. eCollection 2022 Oct. PLoS Pathog. 2022. PMID: 36306287 Free PMC article. - Mechanistic basis for multidrug resistance and collateral drug sensitivity conferred to the malaria parasite by polymorphisms in PfMDR1 and PfCRT.
Shafik SH, Richards SN, Corry B, Martin RE. Shafik SH, et al. PLoS Biol. 2022 May 4;20(5):e3001616. doi: 10.1371/journal.pbio.3001616. eCollection 2022 May. PLoS Biol. 2022. PMID: 35507548 Free PMC article. - Chloroquine and Sulfadoxine-Pyrimethamine Resistance in Sub-Saharan Africa-A Review.
Roux AT, Maharaj L, Oyegoke O, Akoniyon OP, Adeleke MA, Maharaj R, Okpeku M. Roux AT, et al. Front Genet. 2021 Jun 25;12:668574. doi: 10.3389/fgene.2021.668574. eCollection 2021. Front Genet. 2021. PMID: 34249090 Free PMC article. Review.
References
- Bennett, T. N., A. D. Kosar, and P. D. Roepe. 2005. Plasmodium falciparum strain GC-03 exhibits hyper-gametocytogenesis in partially hemoglobin depleted red blood cells. Mol. Biochem. Parasitol. 139:261-265. - PubMed
- Bray, P. G., R. E. Martin, L. Tilley, S. A. Ward, K. Kirk, and D. A. Fidock. 2005. Defining the role of PfCRT in Plasmodium falciparum chloroquine resistance. Mol. Microbiol. 56:323-333. - PubMed
- Bray, P. G., M. Mungthin, I. M. Hastings, G. A. Biagini, D. K. Saidu, V. Lakshmanan, D. J. Johnson, R. H. Hughes, P. A. Stocks, P. M. O'Neill, D. A. Fidock, D. C. Warhurst, and S. A. Ward. 2006. PfCRT and the trans-vacuolar proton electrochemical gradient: regulating the access of chloroquine to ferriprotoporphyrin IX. Mol. Microbiol. 62:238-251. - PMC - PubMed
- Bray, P. G., K. J. Saliba, J. D. Davies, D. G. Spiller, M. R. White, K. Kirk, and S. A. Ward. 2002. Distribution of acridine orange fluorescence in Plasmodium falciparum-infected erythrocytes and its implications for the evaluation of digestive vacuole pH. Mol. Biochem. Parasitol. 119:301-304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources