Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation - PubMed (original) (raw)
Review
Inhibitor of apoptosis proteins in eukaryotic evolution and development: a model of thematic conservation
Mary X D O'Riordan et al. Dev Cell. 2008 Oct.
Abstract
The past decade and a half has witnessed the discovery of a large, evolutionarily conserved family of cellular genes bearing homology to the prototype baculovirus Inhibitor of Apoptosis (IAP). The logical decision in the field to also refer to these cellular proteins as IAPs fails to do justice to this versatile group of factors that play a wide range of roles in eukaryotic development and homeostasis which include, but are not limited to, the regulation of programmed cell death. Here we describe the shared functional characteristics of several well-characterized IAPs whose defining motifs place them more in the category of multifunctional modular protein interaction domains.
Figures
Figure 1. Domain structure of the IAP protein family
The characteristic BIR domains are indicated by red rectangles, CARD domains by purple rectangles, RING domains by green ovals, NBD domains by diamonds, LRR domains by teal circles, and UBC domains by yellow hexagons. DIAP1, DIAP2, Deterin, and dBruce are Drosophila IAPs, while _Sf_IAP1 and _Tn_IAP are lepidopteran IAPs. Abbreviations: IAP, inhibitor of apoptosis; XIAP, X-linked IAP; BIRC, baculoviral IAP repeat containing; hILP, human IAP-like protein; Ts-IAP, testis-specific IAP; c-IAP, cellular IAP; ML-IAP, melanoma-IAP; NAIP, neuronal apoptosis inhibitory protein; DIAP, Drosophila IAP; _Sf_IAP1, Spodoptera frugiperda IAP; _Tn_IAP, Trichoplusia ni IAP; _Ce_BIR-1,-2, Caenorhabditis elegans BIRC _Sp_IAP, Schizosaccharomyces pombe IAP; _Sc_IAP, Saccharomyces cerevisiae IAP; BIR, baculoviral IAP repeat; CARD, caspase recruitment domain; NBD, nucleotide binding oligomerization domain; LRR, leucine rich repeat.
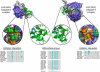
Figure 2. The caspase-binding elements of IAPs
Structure of the complex between XIAP BIR3 and caspase-9 (left, PDB 1NW9) and XIAP BIR2 with caspase-3 (right, PDB 1I3O). The caspase is in surface representation (large subunit in blue, small subunit in grey). The XIAP BIR domain and flanking region are in green cartoon, with the co-ordinated zinc in pink. Caspase inhibition is achieved via two-binding sites: an anchoring interaction with the BIR surface groove, and an “Inhibitory interaction”. Insets: critical XIAP residues that interact with the caspase are in cyan stick representation. Critical caspase residues that interact with XIAP are in orange surface representation. Primary sequence alignment reveals that the BIR surface groove is common to most IAP BIR domains, and there is overlapping binding specificity between IAP BIR domains. The BIR surface groove binds IAP-binding motif (IBM) containing proteins including caspase-9 and -3 (shown here in inset, N-terminus of caspase small subunit in grey stick), caspase-7, Smac/DIABLO, HtrA2/Omi, Grim, Reaper, Hid and others. Alignment of primary sequence across the “Inhibitory interaction” site demonstrates that XIAP is the only IAP that contains all the critical residues to confer direct inhibition of caspase catalytic activity. Although ILP2 also contains all the caspase-9 inhibitory elements, it is an unstable protein whose endogenous expression is yet to be demonstrated.
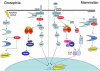
Figure 3. IAP-dependent regulation of conserved Drosophila and mammalian signaling pathways
Inhibition of apoptosis in Drosophila cells by DIAP1 occurs through binding to the initiator and effector caspases, Dronc and DrICE. Similarly, direct binding and inhibition of caspase-3 in mammalian cells is mediated by XIAP. Mammalian c-IAP1 and c-IAP2 can directly bind caspases but are poor caspase inhibitors, instead acting to regulate apoptosis by indirectly modulating caspase-8 activity. Binding of TNF to its receptor results in recruitment of TRADD, RIP, and TRAF2. The cIAPs also participate in pro-survival signaling through TNFR by associating with TRAF2. C-IAP1 and -2 ubiquitinate RIP1, minimizing association with caspase-8 and preventing apoptosis. Additionally the association of RIP, TRAF2, and cIAP1/2 leads to the activation of TAK and subsequent NF-κB and JNK activation, resulting in enhanced transcription of pro-survival genes. C-IAP1 and -2 can also inhibit NIK kinase and downstream processing of p100, thereby negatively regulating NF-κB activation. Thus, the effects of c-IAP1 and -2-dependent signaling on NF-κB are likely context dependent. A TNFR-like pathway regulates immune responses to microbial infection in Drosophila. Peptidoglycan from Gram-negative bacteria is recognized by peptidoglycan recognition proteins (PGRP), which can activate the IMD signaling pathway. IMD is an insect homolog of mammalian RIP1. Genetic studies place IMD, dFADD, Dredd and DIAP2 upstream of or parallel to dTAK activation. DTAK activates both the JNK and Relish pathways analogously to TAK1 in mammalian cells, promoting induction of anti-microbial peptide genes.
Similar articles
- The antiapoptotic activity of insect IAPs requires activation by an evolutionarily conserved mechanism.
Tenev T, Ditzel M, Zachariou A, Meier P. Tenev T, et al. Cell Death Differ. 2007 Jun;14(6):1191-201. doi: 10.1038/sj.cdd.4402118. Epub 2007 Mar 9. Cell Death Differ. 2007. PMID: 17347664 - IAPs: Modular regulators of cell signalling.
Budhidarmo R, Day CL. Budhidarmo R, et al. Semin Cell Dev Biol. 2015 Mar;39:80-90. doi: 10.1016/j.semcdb.2014.12.002. Epub 2014 Dec 24. Semin Cell Dev Biol. 2015. PMID: 25542341 Review. - Inhibitor of apoptosis proteins and apoptosis.
Wei Y, Fan T, Yu M. Wei Y, et al. Acta Biochim Biophys Sin (Shanghai). 2008 Apr;40(4):278-88. doi: 10.1111/j.1745-7270.2008.00407.x. Acta Biochim Biophys Sin (Shanghai). 2008. PMID: 18401525 Review. - Identification of mammalian mitochondrial proteins that interact with IAPs via N-terminal IAP binding motifs.
Verhagen AM, Kratina TK, Hawkins CJ, Silke J, Ekert PG, Vaux DL. Verhagen AM, et al. Cell Death Differ. 2007 Feb;14(2):348-57. doi: 10.1038/sj.cdd.4402001. Epub 2006 Jun 23. Cell Death Differ. 2007. PMID: 16794601 - The ubiquitin ligase ability of IAPs regulates apoptosis.
Ni T, Li W, Zou F. Ni T, et al. IUBMB Life. 2005 Dec;57(12):779-85. doi: 10.1080/15216540500389013. IUBMB Life. 2005. PMID: 16393780 Review.
Cited by
- Drosophila USP5 controls the activation of apoptosis and the Jun N-terminal kinase pathway during eye development.
Fan X, Huang Q, Ye X, Lin Y, Chen Y, Lin X, Qu J. Fan X, et al. PLoS One. 2014 Mar 18;9(3):e92250. doi: 10.1371/journal.pone.0092250. eCollection 2014. PLoS One. 2014. PMID: 24643212 Free PMC article. - Survivin and IAP proteins in cell-death mechanisms.
Altieri DC. Altieri DC. Biochem J. 2010 Sep 1;430(2):199-205. doi: 10.1042/BJ20100814. Biochem J. 2010. PMID: 20704571 Free PMC article. Review. - Interactions between herpesvirus entry mediator (TNFRSF14) and latency-associated transcript during herpes simplex virus 1 latency.
Allen SJ, Rhode-Kurnow A, Mott KR, Jiang X, Carpenter D, Rodriguez-Barbosa JI, Jones C, Wechsler SL, Ware CF, Ghiasi H. Allen SJ, et al. J Virol. 2014 Feb;88(4):1961-71. doi: 10.1128/JVI.02467-13. Epub 2013 Dec 4. J Virol. 2014. PMID: 24307582 Free PMC article. - Targeting autophagy in breast cancer.
Maycotte P, Thorburn A. Maycotte P, et al. World J Clin Oncol. 2014 Aug 10;5(3):224-40. doi: 10.5306/wjco.v5.i3.224. World J Clin Oncol. 2014. PMID: 25114840 Free PMC article. Review. - Modulation of apoptotic response by LAR family phosphatases-cIAP1 signaling during urinary tract morphogenesis.
Stewart K, Tang YC, Shafer MER, Graham-Paquin AL, Bouchard M. Stewart K, et al. Proc Natl Acad Sci U S A. 2017 Oct 24;114(43):E9016-E9025. doi: 10.1073/pnas.1707229114. Epub 2017 Oct 9. Proc Natl Acad Sci U S A. 2017. PMID: 29073098 Free PMC article.
References
- Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat. Med. 1997;3:917–921. - PubMed
- Arama E, Agapite J, Steller H. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev Cell. 2003;4:687–697. - PubMed
- Balachandran S, Thomas E, Barber GN. A FADD-dependent innate immune mechanism in mammalian cells. Nature. 2004;432:401–405. - PubMed
- Bartke T, Pohl C, Pyrowolakis G, Jentsch S. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol Cell. 2004;14:801–811. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous